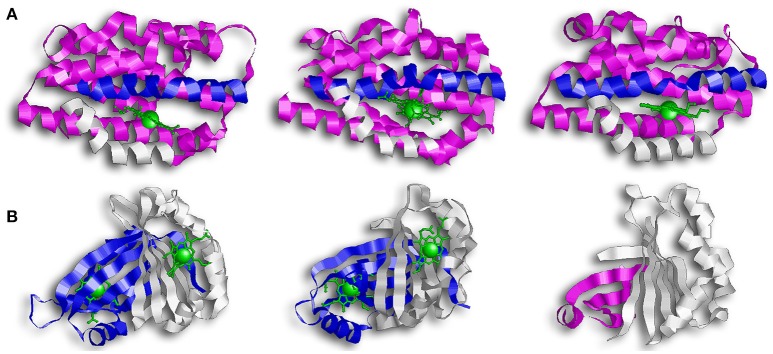
Figure 2.
Overall structure of canonical and IsdG-like heme oxygenases. (A) From left to right: HO-1, HmuO, and PigA. This family of enzymes are commonly referred to as the canonical HOs. They are α-only proteins and have been colored so that the proximal helix is in white and the distal helix is in blue. These helixes take an open conformation when the binding pocket is empty but tighten and close around the heme molecule. Note how the propionate groups in PigA are rotated compared to HO-1 and HmuO. (B) From left to right: IsdG, MhuD, Isd-LmHde. These enzymes represent the second group of HOs, the IsdG-like HOs. This group consists of α/β proteins that dimerize across their β-sheets. Both IsdG and MhuD have been colored so that one monomer is in white and the second monomer is in blue. The Isd-LmHde structure has been colored so that the N-terminal is in magenta and the C-terminal is in white.