Abstract
Background
Cognitive impairment is one of the most important clinical features of neurodegenerative disorders including multiple sclerosis (MS). Conducted research shows that up to 65 percent of MS patients have cognitive deficits such as episodic memory, sustained attention, reduced verbal fluency; however, the cognitive MS domain is information processing speed. It is the first syndrome of cognitive dysfunction and the most widely affected in MS. Occasionally these impairments occur even before the appearance of physical symptoms.
Methods
Therefore, this review focused on the current status of our knowledge about possible methods of treatment cognitive impairment in MS patients including novel strategies. Research and online content was performed using Medline and EMBASE databases.
Results
The most recent research suggests that cognitive impairment is correlated with brain lesion volume and brain atrophy. The examination of the cognitive impairment is usually based on particular neuropsychological batteries. However, it can be not enough to make a precise diagnosis. This creates a demand to find markers that might be useful for identifying patients with risk of cognitive impairment at an early stage of the disease. Currently the most promising methods consist of neuroimaging indicators, such as diffusion tensor imaging, the magnetization transfer ratio, and N-acetyl aspartate levels. Diagnosis problems are strictly connected with treatment procedures. There are two main cognitive therapies: pharmacological (disease modifying drugs (DMD), symptomatic treatments) and non-pharmacological interventions that are focused on psychological and physical rehabilitation. Some trials have shown a positive association between physical activity and the cognitive function.
Conclusion
This article is an overview of the current state of knowledge related to cognition impairment treatment in MS. Additionally, novel strategies for cognitive impairments such as cryostimulation and other complementary methods are presented.
Keywords: Multiple sclerosis, cognitive impairment, pharmacology, non-pharmacological therapies, disease-modifying drugs, neurodegeneration
1. Introduction
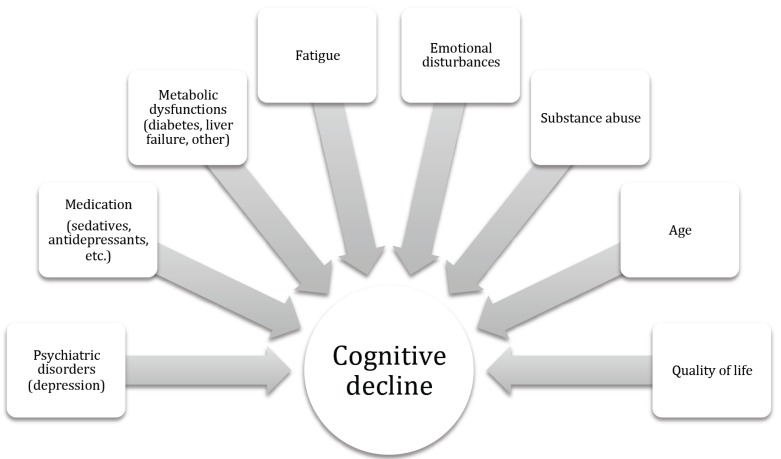
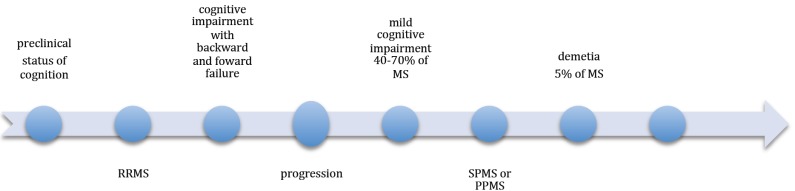
The first attempt to define the pathology and clinical symptoms including cognitive impairment in multiple sclerosis (MS), was made in 1980 by Jean-Martin Charcot, a neurologist [1]. Although, it was the first report about MS as a central nervous system (CNS) disorder, it is still valid. In the early 1950’s neuropsychological testing of MS patients started [2]. Only then, several controlled studies, using standardized and objective neuropsychological measures, confirmed that cognitive dysfunctions might be closely related to MS [3, 4]. Nonetheless, early studies suggested that cognitive deficits are mainly involved in the chronic stage of the disease and secondarily to movement dysfunctions [5]. Since then there has been hardly any data on cognitive dysfunctions in MS. Currently, cognitive impairment is recognized as one of the most common symptoms of many CNS diseases including MS. Therefore, we tried to perform the current status of our knowledge about the possible methods of treatment of cognitive impairment in MS patients including novel strategies. In this review we used Medline and EMBASE databases. MS is the most common neurologic disease disabling young people. Cognition dysfunctions are one of the most important problems MS patients experience in every day routine. For this reason, we summarized achievements in this space of research. It is estimated that approximately 1-2.5 million people all over the world are affected by MS. Women develop MS almost 2.5-times more often than men [6]. Conducted research shows that up to 65 percent of MS patients have cognitive deficits such as episodic memory, sustained attention, reduced verbal fluency; however, the cognitive MS domain determines information processing speed. MS is a complex autoimmune disorder, which manifests itself in the form of destruction of myelin, gradual degeneration of oligodendrocytes and axons, gliosis and some degree of remyelination. Functional impairment in MS patients is connected with many symptoms such as walking problems, poor balance and muscle weakness, which result in axonal degeneration, conduction block and constant micro inflammation processes. The exact etiology of MS is not entirely known [7, 8] but a combination of genetic, infectious, environmental and/or autoimmune factors contribute to the onset of the disease. There are four basic disease patterns in MS: relapsing remitting, primary progressive, secondary progressive and progressive relapsing. Disability in MS progresses gradually and in its advance form, the disease manifests itself with reduced mobility, abnormal gait mechanics, poor balance and muscle weakness, as well as cognitive and autonomic dysfunction [9]. Decreased functional capacity is generally associated with a more severe clinical course. Cognitive deficits are observed in all types and stages of the disease but mainly in the primary and secondary progressive stages [10]. Cognitive decline is usually moderate in MS. The main factors affecting cognitive decline in MS patients are shown in Fig. (1). In older people, primarily women, the symptoms can be more severe. However, the significant dementia is very rare [11]. Disability and cognitive dysfunction do not develop parallel in MS. Development of cognitive impairment in MS is unpredictable (Fig. 2). Moreover, both disability level and disease duration are not the predictors of the degree of cognitive dysfunction [12]. It was suggested that cognitive impairment is different in particular individuals according to the history of MS as well as lesion volume and location in the brain . There are several cognitive domains, specific and characteristic for MS. The most common are: learning and memory, speed of information processing, attention and executive functioning. Whereas, language ability (repetition, comprehension, fluency) is atypical for MS [13]. MS is the most common cause of disability in young adults. Although, the pathogenesis of MS is not entirely known, it is described as inflammatory demyelinating disease and CNS axonal damage correlates with a degree of inflammatory processes [14-16]. Differential changes in the conduction of nervous impulse by demyelinated motor and sensory tracts within the CNS can alter functional and mental status. Individual functional capacity, disease progression and symptom management influence MS and its impact on patient’s disability and quality of life concomitant with pharmacological agents. The patient’s specific features and disease related factors are different in distinct stages of MS. Therefore, the treatment, which is effective in one group of MS patients, might be deleterious in another. In order to implement an MS therapy, tailored for a particular patient, or create future, there is a need to define specific clinical parameters to differentiate pathological patterns during the patient’s lifespan [17]. Magnetic resonance imagining (MRI) allows detect microstructural changes in tissue compartments. MRI basic methods include diffusion tensor imaging (DTI) and magnetization transfer imaging (MTI). Frazekas et al. (2005) reported significant decrease in magnetization transfer ratio (MTR) in normal white matter (NAWM) and cortex in elderly people. The age-related effect was the strongest in frontal and parieto-occipital cortical regions of the brain [18]. Draganski et al. (2011) observed an age-dependent MTR decrease in the frontal cortex and almost a linear dose-effect relationship between results in all cognitive domains and the quartile distribution of MTR in the brain cortex. Lower distribution of MTR in deep gray matter was related to executive dysfunction [19]. Moreover, it is concomitant with the hypothesis that the regional pattern of MTI-detected microstructural CNS tissue changes determines the pattern of cognitive dysfunctions. Executive dysfunction is in relation to frontal-subcortical circuits lesions [20]. Demyelination is an age-related process and it is observed very often in elderly people as a consequence of cerebral small vessel disease [21, 22]. Benedict et al. (2004) [23] reported a relation between neocortical volume and variety of neuropsychological tests (verbal and visuospatial, memory, processing speed, and working memory). Moreover, they observed a correlation between cognitive impairment and increased volume of the third ventricle [23, 24], probably related to thalami atrophy [25]. Summers et al. (2008) made an observation that the reduction in cerebral volume in one year of progression results in cognitive impairment, which remains for 5 years [26]. Additionally, there is a strict correlation between overall cortical thickness and cognitive impairment [27, 28]. Sanfilipo et al. (2005) [29] studied the different role of WM and GM in cognition process. Their research suggested that there is an association between WM loss and dysfunction of processing speed and working memory, whereas GM mainly contributes to verbal memory. Moreover, there are studies suggesting that the patterns of GM atrophy are similar in MS patients both with fatigue and cognitive impairments [28] in contrast to weak correlation between these symptoms and WM lesion. These pathological changes in brain can contribute not only to cognitive difficulties but also to fatigue problems and depression.
Fig. (1).
The main factors affecting cognitive decline in multiple sclerosis patients.
Fig. (2).
Development of cognitive impairment in multiple sclerosis (MS).
1.1. Cognitive Fatigue
Approximately 65-70% of individuals with MS demonstrate fatigue symptoms and about 40% claim fatigue to be a crucial feature, which is a more severe cause of their disability than age, loss of muscle strength, spasticity, balance or bowel-bladder problems [6, 30]. MS fatigue occurs in various forms, but very often it is felt as general tiredness or lassitude even without any physical effort [31]. Although, systemic fatigue is different; it highly negatively affects everyday activities. Moreover, there is also cognitive fatigue that appears as reduced attention, memory and/or information processing distributions. The processes, which contribute to a decrease in muscle strength, include lower motor unit firing rates, reduced motor unit recruitment as well as extended conduction time in neurons. Furthermore, peripheral changes also occur in muscle weakness such as atrophy, decrease in oxidative capacity and increased production of energy in anaerobic metabolism [32].
1.2. Depression
Depression and anxiety are one of the most common mental problems in MS patients. Approximately, 50% of MS patients experience depression symptoms. Moreover, the risk of suicide in MS is much higher (even 7.5-times) than in the general population. Depression may develop both in the onset and in progressive stage of MS [33]. MS is a multifactorial, complex disorder, so it can affect not only the CNS but also the neuroendocrine system responsible for emotion and contribute to emotional liability [34]. Moreover, standard pharmacological treatment, especially steroid therapy, might also be involved in mental changes including depression, being an adverse effect of some drugs. Currently, depression as well as fatigue is treated with pharmacological and non-pharmacological therapies. One of the most often studied therapies is aerobic training [35]. However, there are also other methods such as: cryostimulation, yoga, especially in mild depression.
2. Standard MS treatment
The therapeutic options of treatment relapsing forms of MS have significantly expanded over the past two decades. With the introduction of monoclonal antibody treatments, multiple highly effective options are available to help reduce the risk of disability in MS patients. The following disease-modifying drugs (DMD), approved by the U.S. Food and Drug Administration (FDA), allow reducing disease activity and progression in patients with relapsing-remitting stage of disease, as well as progressive stages in those patients who still experience relapses. DMD is a standard treatment in MS. A relapse is defined as an occurrence of one or several symptoms or exacerbation of the clinical status in MS patient lasting over 24 hours. This new status is observed not earlier than within one month following a previous exacerbation [36]. The drug description according to the most current version of National MS Society and its potential role in cognition functioning are shown in Table 1.
Table 1.
The standard disease modifying therapies for multiple sclerosis (MS) according to National MS Society (2015).
| Drug |
Dose and Route of
Administration |
FDA Approval | Recommendation to MS Clinical Course | Drug Description |
Cognitive
Enhancer |
|
|---|---|---|---|---|---|---|
| Interferon beta-1a |
30mcg/once a week (intramuscular) |
(1996) Avonex |
RRMS CIS SPMS |
Glycoprotein | +/- | Ref. |
| - | - | |||||
| 22mcg or 44mcg 3xweek (subcutaneously) | (2002) Rebif |
+ | [37] [38] |
|||
| Interferon beta-1b |
0.26mg/day (subcutaneously) |
(1993) Betaferon |
RRMS CIS SPMS |
Protein recombinatly produced by Escherichia coli | + | [39] [40] |
| Glatiramer acetate | 20mg /day or 40mg 3xweek (subcutaneously) | (2009) Copaxone |
RRMS CIS |
Copolymer1 of myelin basic protein (glutamid acid,lysine, alanine, tyrosine) | + | [40] |
| Daclizumab | 150mg once a month (intravenously) |
(2016) Zenapax |
RRMS | Humanized monoclonal antibody with IgG1 framework. Originaly used as a prevention allogenic tissue transplantation |
- | - |
| Terilunomide | 7mg or 14mg /day(oral) | (2012) Aubagio |
RRMS | Metabolite of leflunomide | - | - |
| Dimethyl fumarate | 120mg /2xday for one week, 240mg capsule taken daily (oral) | (2013) Tecfidera |
RRMS CIS |
Furmate (BG0012) immunomodulatory agent |
- | - |
| Fingolimod | 0.5mg/day (oral) |
(2010) Gineya |
RRMS | Structural analogue of sphingosine | - | - |
| Alemtuzumab | 12mg/day for 5 days, next 12mg/day for 3 days in the next year(intravenously) |
(2014) Lemtrada |
RRMS | Humanized monoclonal antibody. Originally used in B-cell chronic lymphocytic leukemia. Reserved for people who have had an inadequate response to two or |
- | - |
| Mitoxantrone | 12mg/m(2) every 3 months. About 8012 doses over 2-3 years (140mg/m(2)) (intravenously) | (2000) Novantrone |
SPMS; PRMS or worsening RRMS | A synthetic anthracenedione that intercalates into DNA | - | - |
| Natalizumab | 300mg once every 28days (intravenously) |
(2006) Tysabri |
RRMS | Humanized monoclonal antibody with an IgG4 framework | + | [42] [43] |
3. The influence of MS treatment on cognition
There are data that DMDs can improve results by reducing the number of lesion cases, functional status or relapse rate. Moreover, the outcomes of MRI such as lesion load and brain volume appear to correlate with the level of cognitive impairment. Current studies suggest that the DMDs also affect cognition. The clinical study, COGIMUS (cognitive impairment in multiple sclerosis) has revealed cognitive decline in the RRMS group of patients treated with Interferon beta-1a, administered subcutaneously in two different doses 22 and 44 μg (SC) 3x/week for 3 years. It was reported that
IFN-beta 1a might have a positive impact on cognition; especially in the group of MS patients who received the 44 μg (the cognitive decline was reduced by 32%) [37, 38]. Moreover, 265 patients participated in two-year follow-up examination. Results confirm an earlier observation but it should be noted that the women achieve better results in protection against cognitive impairment. Barak and Achrion reported that INFbeta-1b might improve cognitive functioning [39]. These observations were confirmed by the multicenter study on a group of 161 MS patients with cognition problems. The analyses were made using the brief International Cognitive Assessment (BICAMS) battery and monitoring the first line of DMDs. The outcomes after 1 year showed significant positive effects in terms of cognitive status between MS patients using IFNB and Glatiramer Acetate and healthy controls [40]. Another quite a new MS drug is Natalizumab (humanized monoclonal antibody), which is used as monotherapy in severe clinical courses. Clinical studies, one of which TOP (Tysabri Observational Program) confirmed the positive effect of Natalizumab therapy on functional status, level of disability, rate of relapses [41]. Currently, there are several longitude studies, which confirmed the positive impact of natalizumab therapy on cognition, depression and fatigue. For example, Mattoli et al. (2015) reported that after 3 years of administering Natalizumab both the number of pathological tests and single test performances in attention (PASAT), executive functions (WCST) and memory functions (Short tale and Rey figure recall) have significantly improved [42]. Moreover, the best outcomes were reported after the first year of therapy, especially in MS patients with lower level of disability. Natalizumab shows a neuroprotective role as it reduces the inflammatory processes in the brain, such as atrophy, being a key factor as well as improves cognitive functioning. MS immunomodulatory treatment mostly involves restraining of brain damage in the early phase of the disease [43, 44]. Another common problem among MS patients is depression. Fatigue and depression are very difficult to differentiate since certain symptoms are similar in both the diseases. It is known that IFN beta-1b therapy can exacerbate depression, especially within about 6 months following its onset. About 41% of MS patients demonstrated exacerbation of depression symptoms or occurrence of new ones [45]. Effects of symptomatic therapies such as modafinil and donepezil are inconsistent. Most studies whose authors obtained positive findings, demonstrate significant methodological problems, which makes it difficult to propose proper treatment recommendations. There are no published reports on fingolimod that appeared to be effective in improving cognition in controlled trials. Application of DMD in other forms of MS and clinically isolated syndrome has not yielded positive results. There are hardly any data regarding a relationship between behavioral therapy, symptomatic treatment or DMD and reduction of cognitive decline or improvement of impaired cognition. Treatment and prevention of cognitive impairment need to remain key research foci, as it can contribute to identification of new interventions and improvement of clinical trial methodology [46].
4. Pharmacological cognitive enhancer
Currently, there are many therapies (drugs, supplements, nutraceuticals, and functional foods) or methods such as brain stimulation, yoga, aerobic trainings, cryostimulation called Cognitive Enhancers (CE). There are pharmacological (PCE) and Non-Pharmacological Cognitive Enhancers (NPCE). PCEs include herbal medicines, gingko biloba, and Nootropil, curcuma etc.) and pharmaceutical drugs (acetylcholinesterase inhibitors or memantine). Cognitive enhancing drugs, such as cholinesterase inhibitors and methylphenidate, are used to improve cognitive functioning in neurodegenerative disorders such as Alzheimer disease or other CNS issues manifesting themselves with deficit of attention and hyperactivity. However, these drugs including modafinil, are used by healthy people for enhancement purposes [47]. Pharmacological therapies of comorbidities including depression or fatigue can also have positive effect, but now there is no consistent evidence in this field of research. MS is a complex disease with variety of symptoms. Therefore, the use of cognitive enhancer could provide some benefits in contrasting to side effects of other drugs such as baclofen, benzodiazepines etc.
4.1. Acetylcholinesterase Inhibitors (AChEI)
Recent studies on AChEI included studies on donepezil because this drug appears to be well tolerated by MS patients. However, current clinical research is very limited and insufficient. Hence, it is not advisable to recommend AChEI as a pharmaceutical drug in therapy of cognitive impairment in MS. The largest randomized controlled trial of donepezil included 69 MS patients. The authors of the study reported a significant improvement in verbal learning and memory compared with a placebo group during neuropsychological testing [48]. However, there are still many questions about the application of AChEIs in MS, including side effects after long-term use [49]. Another multicenter, double-blind with placebo study, has been published by Krupp et al. (2011). This clinical study included one hundred and twenty MS patients and no significant treatment effect was found [50].
4.2. Memantine
Similar findings were observed with regards to recommending the use of memantine in the treatment of cognitive impairment in MS patients. Only two studies have been published: 60 MS patients participated in one of the studies [51], but due to exacerbation of neurological symptoms the studies were prematurely discontinued. The other research on a group of 126 MS patients showed negative outcomes of memantine therapy [52].
5. Non-pharmacological cognitive treatment
Currently, there are many additional therapies (supplements, nutraceuticals, and functional foods) or methods such as brain stimulation, yoga, aerobic trainings named Cognitive Enhancers (CE). There are pharmacological (PCE) and Non-Pharmacological Cognitive Enhancers (NPCE). PCE include herbal medicines (asparagus, gingko biloba, and Nootropil, curcuma etc.) and pharmaceutical drugs whose treatment recommendation criteria are insufficient [53]. Therefore, NPCE are recommended due to their high safety profile and low-cost methods, especially physical exercise, sleep, meditation, computer training, brain stimulation, yoga or music [54].
5.1. Sleep
Sleep is key factor for effective brain functioning. Lack of adequate sleep is the main reason for a great number of cognitive dysfunctions such as attention and memory, language and reasoning working memory, language processing, creativity and decision-making. Many studies confirmed that short time naps during a day have positive effect on memory performance and concentration, integration and reprocessing of fresh memories into the existing reservoir of long-term memories.
Poor quality sleep can appear long before any cognitive symptoms in the early or preclinical phase of MS. Sleep disturbances are associated with more severe cognitive decline and higher risk of mild cognitive impairment. Cognition declines tend to be more active in SPMS and PPMS patients and correlates with chronic brain tissue damage, including neuroaxonal degeneration, white matter lesion volume and cortical atrophy [13, 54].
5.2. Aerobic Training
MS patients demonstrate a lower level of physical activity than healthy people. Therefore, aerobic training (AT), can be very important factor preventing decondition, osteoporosis, cardiovascular problems, obesity and other MS specific symptoms [55]. Obtained data, suggest that exercises, mainly aerobic ones, can improve cognitive functioning. Cognition-enhancing effects of AT were studied in both in vivo and animal experiments. Positive effect of AT on physiological processes such as glucoregulation and cardiovascular health is well known [56]. Several clinical studies revealed some benefits for executive control including planning, selective attention, multitasking work, and inhibition, working memory, particularly in women. Brain imaging studies show that AT might be associated with reduced age-related atrophy and more effective perfusion in brain regions responsible for executive control and memory. Last, clinical studies showed that AT may increase hippocampal volume due to an increased level of brain-derived neurotrophic factor which is one of the most important molecules affecting neurogenesis and stimulating dendritic network [57].
5.3. Neuropsychological Rehabilitation
The aim of neuropsychological rehabilitation is reducing cognitive impairment as well as increase patients awareness of cognition deficits in everyday activities. Neuroplasticity due to remyelinisation processes may reduce the cognitive decline. Cognitive rehabilitation is typically a combination of various methods, tailored to the patient’s individual needs. Therefore the effectiveness of the multifactorial intervention is difficult to evaluate. High-quality studies on a relationship between rehabilitation and improvement of cognition in MS patients have revealed hardly any evidence. A recent meta-analysis of randomized and quasi-randomized trials showed that cognitive training can increase memory span and working memory [35]. It further indicated that cognitive training, combined with other neuropsychological methods, can improve attention, immediate verbal memory, and delayed memory. Last randomized controlled trial, conducted by Hanssen et al. (2016), investigated positive effects of multicomponent cognitive rehabilitation in a group of 120 MS patients with cognitive complaints in psychological aspects of HRQoL [58]. In the recent Cochran review the impact of psychological therapies on cognition improvement in MS patients was presented. However, it was found that published literature show only low-level of evidence [59]. Cognitive rehabilitation is an additional clinical practice used in MS patients with possitive subjective assessement. Current status of knowledge is not sufficient to establish specific recommendation to neuropsychological rehabilitation in MS patients. Therefore, future research should be condacted including novel therapies.
6. Novel therapies
6.1. Whole Body Cryostimulation (WBC)
Whole body cryostimulation (WBC) is a new therapy used mainly as biological regeneration primarily in sports medicine. Currently, WBC is a therapy aiming to reduce inflammation, pain in many disorders such as fibromyalgia, sports injury, osteoarthritis and others. Recent studies suggest that it can be promising additional therapy in MS patients with fatigue. However, there are only a few data about WBC therapy in MS. In our last study, we analyzed two groups of MS patients with fatigue syndrome: low-fatigue (score rate 38-42 in the Fatigue Severity Scale FSS) and high-fatigue (48-52 FSS). After 10 sessions of WBC exposition we observed improvement in the feeling of fatigue in both groups but significantly greater in high-fatigue MS patients [60]. A patient exposed to WBC in a special cryogenic chamber with liquid nitrogen as a coolant usually 10 times (1 exposure per day). There are two rooms: a vestibule, with a temperature of -60°C, and the main chamber, with temperatures about -110 °C. One session in the cryogenic chamber lasts 2–3 min.
6.2. Brain Stimulation Techniques
Brain stimulation techniques were used in psychiatry or neurology. There are non-invasive methods such as Transcranial Direct Current Stimulation (tDCS) and Transcranial Magnetic Stimulation (TMS) and invasive ones, including Deep Brain Stimulation (DBS) and direct vagus nerve stimulation (dVNS) [61].
Most of these therapies are aimed to help in encoding memory and learning processes; others like DBS may directly alter and affect memory systems [62]. TMS and tDCS are the most often studied. Studies indicate that TMS and tDCS stimulation methods of the anterior temporal lobe help to enhance speed of recall [63, 64]. Moreover, tDCS can enhance performance of working memory tasks, learning and recall of words during stimulation of the left dorso-lateral prefrontal cortex (DLPFC) during encoding [65]. TMS and tDCS are suggested as therapies that can enhance cognitive skills due to stimulation of brain plasticity processes and facilitating of learning motor tasks by activation motor areas needed to rehabilitation. Headaches, local pain, confusion and occurrence of seizure are the most common side effects after or during a performance of brain stimulation techniques.
Application of rTMS and its impact on cognition in depressed patients have brought conflicting outcomes [66]. However, a few studies report improvement in cognitive functions after the use of rTMS over the left DLPFC [67, 68]. Long-lasting DBS in MS patients improved tremor control and quality of life [69].
6.3. Computer Based Training Programs
Computer-based repetitive stimulation is a new therapy allows to train many cognitive tasks. Computer-based training programs have proved to be additional therapies that can improve memory, attention, executive function and processing speed. They can be administered both in the young and the elderly [70] to prevent dementia and age-related cognitive impairment [71]. Computer-based training has different effects in specific cognitive domains. Great effects were observed in processing speed and perceptual measures, while small or medium ranged effects were noted in memory domains [70]. However, Whitlock et al. (2012), indicated no effects of game-based cognitive training on visuo-spatial navigational abilities and memory [72] with negative patients behavior such as aggression and reduction of empathy especially in violent computer games [73]. Computer- based cognitive training is mainly used in mild cognitive impairments as additional therapy mainly in verbal learning and memory [74, 75].
Conclusion
Cognitive dysfunction is a core feature of MS. Cognitive impairment is observed in all subtypes but most often in secondary progressive (SP) and primary progresssive (PP). In MS patients mild to moderate cognitive decline is mainly observed. Dementia is very rare in MS (about 5%). The changes in MRI parameters of WM lesion volume and cortical atrophy are the best predictors of cognitive dysfunction. MS is a complex disease with many clinical and pathological factors which can contribute to cognition functioning including depression, fatigue as well as fluctuation of the inflammation or activity of immunological processes. MRI techniques presented in many studies facilitate understanding of these processes. Results of MRI studies suggest that both WM and GM injuries are involved in cognitive impairment. However, WM damage might change connectivity inside the neuronal networks and in this way, it may reduce processing speed, attention and working memory. On the other hand, GM injury may result in memory changes and altered behavior. Currently there is still lack of phamacological cognitive enhancers with high level of effectivness, but some promising outcomes in non-pharmacological approach were reported. Cognitive problems are sometimes invisible but difficult for MS patients. Rehabilitation is recommended for all stages of MS, but the best results were observed at the early stage. Patients with MS have individual and wide-ranging needs so rehabilitation is usually provided by multidisciplinary team. Pharmacological therapies for reducing disease activity in MS significantly expanded over the last year. However, no effective treatment has been established in the case of cognitive problems. Therefore, there is a need to find new strategies, which can be used especially at an early stage of MS to prevent cognition decline.
Consent for Publication
Not applicable.
Acknowledgements
We thank all of our colleagues in the Neurorehabilitation Ward in the III General Hospital of Lodz, Poland.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Charcot J.M. 1880. Lecons sur les maladies du systeme nerveux faites a la Salpe-triere,1872sc ed.; A. Delahaye: Paris, [Google Scholar]
- 2.Ross A.T., Reitan R.M. Intellectual and affective functions in multiple sclerosis; a quantitative study. AMA Arch. Neurol. Psychiatry. 1955;73(6):663–677. doi: 10.1001/archneurpsyc.1955.02330120067007. [http://dx.doi.org/10.1001/archneurpsyc. 1955.02330120067007]. [PMID: 14375430]. [DOI] [PubMed] [Google Scholar]
- 3.Langdon D., Holloway R. Introduction to cognition and MS. Cognition and MS; 2013. pp. 4–18. [Google Scholar]
- 4.Morel A., Bijak M., Miller E., Rywaniak J., Miller S., Saluk J. Relationship between the increased haemostatic properties of blood platelets and oxidative stress level in multiple sclerosis patients with the secondary progressive stage. Oxyd. Med. Cell. Longev., 2015. [DOI] [PMC free article] [PubMed]
- 5.Brissart H., Morele E., Baumann C., Perf M.L., Leininger M., Taillemite L., Dillier C., Pittion S., Spitz E., Debouverie M. Cognitive impairment among different clinical courses of multiple sclerosis. Neurol. Res. 2013;35(8):867–872. doi: 10.1179/1743132813Y.0000000232. [http://dx.doi.org/ 10.1179/1743132813Y.0000000232]. [PMID: 23816638]. [DOI] [PubMed] [Google Scholar]
- 6.Rogers J.M., Panegyres P.K. Cognitive impairment in multiple sclerosis: evidence-based analysis and recommendations. J. Clin. Neurosci. 2007;14(10):919–927. doi: 10.1016/j.jocn.2007.02.006. [http://dx.doi.org/10.1016/j.jocn. 2007.02.006]. [PMID: 17659875]. [DOI] [PubMed] [Google Scholar]
- 7.Miller E. Multiple sclerosis. Adv. Exp. Med. Biol. 2012;724:222–238. doi: 10.1007/978-1-4614-0653-2_17. [http://dx.doi.org/10.1007/978-1-4614-0653-2_17]. [PMID: 22411246]. [DOI] [PubMed] [Google Scholar]
- 8.Peedicayil J. Epigenetic Drugs for Multiple Sclerosis. Curr. Neuropharmacol. 2016;14(1):3–9. doi: 10.2174/1570159X13666150211001600. [http://dx.doi.org/10.2174/ 1570159X13666150211001600]. [PMID: 26813117]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantarci O., Wingerchuk D. Epidemiology and natural history of multiple sclerosis: new insights. Curr. Opin. Neurol. 2006;19(3):248–254. doi: 10.1097/01.wco.0000227033.47458.82. [http://dx.doi.org/10.1097/01.wco.0000227033.47458.82]. [PMID: 16702830]. [DOI] [PubMed] [Google Scholar]
- 10.Dalgas U., Stenager E., Ingemann-Hansen T. Multiple sclerosis and physical exercise: recommendations for the application of resistance-, endurance- and combined training. Mult. Scler. 2008;14(1):35–53. doi: 10.1177/1352458507079445. [http://dx.doi.org/10.1177/1352458507079445]. [PMID: 17881393]. [DOI] [PubMed] [Google Scholar]
- 11.Staff N.P., Lucchinetti C.F., Keegan B.M. Multiple sclerosis with predominant, severe cognitive impairment. Arch. Neurol. 2009;66(9):1139–1143. doi: 10.1001/archneurol.2009.190. [http://dx.doi.org/10.1001/archneurol.2009.190]. [PMID: 19752304]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foley J.F., Brandes D.W. Redefining functionality and treatment efficacy in multiple sclerosis. Neurology. 2009;72(23) Suppl. 5:S1–S11. doi: 10.1212/WNL.0b013e3181a99bc2. [http://dx.doi.org/10.1212/WNL.0b013e3181a99bc2]. [PMID: 19506262]. [DOI] [PubMed] [Google Scholar]
- 13.Bartko D., Čombor I., Kubovičova K., Gombošová Z. Multiple sclerosis and cognitive disorders. What should neurologists advice patient with MS about his risk of developing dementia. Act. Nerv. Super. Rediviva. 2012;54:143–149. [Google Scholar]
- 14.Frischer J.M., Bramow S., Dal- Bianco A., Lucchinetti C.F., Rauschka H., Schmidbauer M., Laursen H., Sorensen P.S., Lassmann H. The relation between inflammation and neu – rodegeneration in multiple sclerosis brains. Brain. 2009;132:1175–1189. doi: 10.1093/brain/awp070. [http://dx.doi.org/10.1093/brain/awp070]. [PMID: 19339255]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bethoux F., Bennett S. Evaluating walking in patients with multiple sclerosis: which assessment tools are useful in clinical practice? Int. J. MS Care. 2011;13(1):4–14. doi: 10.7224/1537-2073-13.1.4. [http://dx.doi.org/10.7224/1537-2073-13.1.4]. [PMID: 24453700]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbar A., Bahadoran R., Ghasemzadeh Y. The effect of aquatic exercise on balance of adults with multiple sclerosis. Eur. J. Exp. Biol. 2014;4:38–43. [Google Scholar]
- 17.Lucchinetti C., Brück W., Parisi J., Scheithauer B., Rodriguez M., Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 2000;47(6):707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [http://dx.doi.org/10.1002/1531-8249(200006) 47:6<707:AID-ANA3>3.0.CO;2-Q]. [PMID: 10852536]. [DOI] [PubMed] [Google Scholar]
- 18.Fazekas F., Ropele S., Enzinger C., Gorani F., Seewann A., Petrovic K., Schmidt R. MTI of white matter hyperintensities. Brain. 2005;128(Pt 12):2926–2932. doi: 10.1093/brain/awh567. [http://dx.doi.org/10.1093/ brain/awh567]. [PMID: 15958507]. [DOI] [PubMed] [Google Scholar]
- 19.Draganski B., Ashburner J., Hutton C., Kherif F., Frackowiak R.S., Helms G., Weiskopf N. Regional specificity of MRI contrast parameter changes in normal ageing revealed by voxel-based quantification (VBQ). Neuroimage. 2011;55(4):1423–1434. doi: 10.1016/j.neuroimage.2011.01.052. [http://dx.doi.org/10.1016/j.neuroimage.2011.01.052]. [PMID: 21277375]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krause M., Mahant N., Kotschet K., Fung V.S., Vagg D., Wong C.H., Morris J.G. Dysexecutive behaviour following deep brain lesions--a different type of disconnection syndrome? Cortex. 2012;48(1):97–119. doi: 10.1016/j.cortex.2011.03.014. [http://dx.doi.org/10.1016/j.cortex.2011. 03.014]. [PMID: 21546014]. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt R., Schmidt H., Haybaeck J., Loitfelder M., Weis S., Cavalieri M., Seiler S., Enzinger C., Ropele S., Erkinjuntti T., Pantoni L., Scheltens P., Fazekas F., Jellinger K. Heterogeneity in age-related white matter changes. Acta Neuropathol. 2011;122(2):171–185. doi: 10.1007/s00401-011-0851-x. [http://dx.doi.org/10.1007/s00401-011-0851-x]. [PMID: 21706175]. [DOI] [PubMed] [Google Scholar]
- 22.Seiler S., Pirpamer L., Hofer E., Duering M., Jouvent E., Fazekas F., Mangin J.F., Chabriat H., Dichgans M., Ropele S., Schmidt R. Magnetization transfer ratio relates to cognitive impairment in normal elderly. Front. Aging Neurosci. 2014;6:263. doi: 10.3389/fnagi.2014.00263. [http://dx.doi.org/10.3389/fnagi.2014.00263]. [PMID: 25309438]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benedict R.H., Cox D., Thompson L.L., Foley F., Weinstock-Guttman B., Munschauer F. Reliable screening for neuropsychological impairment in multiple sclerosis. Mult. Scler. 2004;10(6):675–678. doi: 10.1191/1352458504ms1098oa. [http://dx.doi.org/10.1191/1352458504ms1098oa]. [PMID: 15584493]. [DOI] [PubMed] [Google Scholar]
- 24.Tiemann L., Penner I.K., Haupts M., Schlegel U., Calabrese P. Cognitive decline in multiple sclerosis: impact of topographic lesion distribution on differential cognitive deficit patterns. Mult. Scler. 2009;15(10):1164–1174. doi: 10.1177/1352458509106853. [http://dx.doi.org/10.1177/ 1352458509106853]. [PMID: 19667010]. [DOI] [PubMed] [Google Scholar]
- 25.Houtchens M.K., Benedict R.H., Killiany R., Sharma J., Jaisani Z., Singh B., Weinstock-Guttman B., Guttmann C.R., Bakshi R. Thalamic atrophy and cognition in multiple sclerosis. Neurology. 2007;69(12):1213–1223. doi: 10.1212/01.wnl.0000276992.17011.b5. [http://dx.doi.org/10.1212/01.wnl. 0000276992.17011.b5]. [PMID: 17875909]. [DOI] [PubMed] [Google Scholar]
- 26.Summers M., Swanton J., Fernando K., Dalton C., Miller D.H., Cipolotti L., Ron M.A. Cognitive impairment in multiple sclerosis can be predicted by imaging early in the disease. J. Neurol. Neurosurg. Psychiatry. 2008;79(8):955–958. doi: 10.1136/jnnp.2007.138685. [http://dx.doi.org/10.1136/ jnnp.2007.138685]. [PMID: 18339729]. [DOI] [PubMed] [Google Scholar]
- 27.Amato M.P., Goretti B., Ghezzi A., Lori S., Zipoli V., Portaccio E., Moiola L., Falautano M., De Caro M.F., Lopez M., Patti F., Vecchio R., Pozzilli C., Bianchi V., Roscio M., Comi G., Trojano M. Cognitive and psychosocial features of childhood and juvenile MS. Neurology. 2008;70(20):1891–1897. doi: 10.1212/01.wnl.0000312276.23177.fa. [http://dx.doi. org/10.1212/01.wnl.0000312276.23177.fa]. [PMID: 18474844]. [DOI] [PubMed] [Google Scholar]
- 28.Calabrese M., Filippi M., Gallo P. Cortical lesions in multiple sclerosis. Nat. Rev. Neurol. 2010;6(8):438–444. doi: 10.1038/nrneurol.2010.93. [http://dx.doi.org/ 10.1038/nrneurol.2010.93]. [PMID: 20625376]. [DOI] [PubMed] [Google Scholar]
- 29.Sanfilipo M.P., Benedict R.H., Sharma J., Weinstock-Guttman B., Bakshi R. The relationship between whole brain volume and disability in multiple sclerosis: a comparison of normalized gray vs. white matter with misclassification correction. Neuroimage. 2005;26(4):1068–1077. doi: 10.1016/j.neuroimage.2005.03.008. [http://dx.doi.org/10.1016/j.neuroimage.2005. 03.008]. [PMID: 15961046]. [DOI] [PubMed] [Google Scholar]
- 30.McIntosh-Michaelis S.A., Roberts M.H., Wilkinson S.M., Diamond I.D., McLellan D.L., Martin J.P., Spackman A.J. The prevalence of cognitive impairment in a community survey of multiple sclerosis. Br. J. Clin. Psychol. 1991;30(Pt 4):333–348. doi: 10.1111/j.2044-8260.1991.tb00954.x. [http:// dx.doi.org/10.1111/j.2044-8260.1991.tb00954.x]. [PMID: 1777755]. [DOI] [PubMed] [Google Scholar]
- 31.Khan F., Amatya B., Galea M. Management of fatigue in persons with multiple sclerosis. Front. Neurol. 2014;5:177. doi: 10.3389/fneur.2014.00177. [http://dx. doi.org/10.3389/fneur.2014.00177]. [PMID: 25309504]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giovannoni G. Multiple sclerosis related fatigue. J. Neurol. Neurosurg. Psychiatry. 2006;77(1):2–3. doi: 10.1136/jnnp.2005.074948. [http://dx.doi.org/10.1136/ jnnp.2005.074948]. [PMID: 16361582]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chwastiak L.A., Ehde D.M. Psychiatric issues in multiple sclerosis. Psychiatr. Clin. North Am. 2007;30(4):803–817. doi: 10.1016/j.psc.2007.07.003. [http://dx. doi.org/10.1016/j.psc.2007.07.003]. [PMID: 17938046]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patten S.B., Neutel C.I. Corticosteroid-induced adverse psychiatric effects: incidence, diagnosis and management. Drug Saf. 2000;22(2):111–122. doi: 10.2165/00002018-200022020-00004. [http://dx.doi.org/10.2165/00002018-200022020-00004]. [PMID: 10672894]. [DOI] [PubMed] [Google Scholar]
- 35.Karakaya T., Fußer F., Schröder J., Pantel J. Pharmacological treatment of mild cognitive impairment as a prodromal syndrome of Alzheimer’s disease. Curr. Neuropharmacol. 2013;11(1):102–108. doi: 10.2174/157015913804999487. [PMID: 23814542]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loma I., Heyman R. Multiple sclerosis: pathogenesis and treatment. Curr. Neuropharmacol. 2011;9(3):409–416. doi: 10.2174/157015911796557911. [http://dx.doi. org/10.2174/157015911796557911]. [PMID: 22379455]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patti F., Morra V.B., Amato M.P., Trojano M., Bastianello S., Tola M.R., Cottone S., Plant A., Picconi O. Subcutaneous interferon β-1a may protect against cognitive impairment in patients with relapsing-remitting multiple sclerosis: 5-year follow-up of the COGIMUS study. PLoS One. 2013;8(8):e74111. doi: 10.1371/journal.pone.0074111. [http://dx.doi. org/10.1371/journal.pone.0074111]. [PMID: 24137499]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patti F., Amato M.P., Trojano M., Bastianello S., Tola M.R., Goretti B., Caniatti L., Di Monte E., Ferrazza P., Brescia Morra V., Lo Fermo S., Picconi O., Luccichenti G. Cognitive impairment and its relation with disease measures in mildly disabled patients with relapsing-remitting multiple sclerosis: baseline results from the Cognitive Impairment in Multiple Sclerosis (COGIMUS) study. Mult. Scler. 2009;15(7):779–788. doi: 10.1177/1352458509105544. [http://dx.doi.org/10. 1177/1352458509105544]. [PMID: 19542262]. [DOI] [PubMed] [Google Scholar]
- 39.Barak Y., Achiron A. Effect of interferon-beta-1b on cognitive functions in multiple sclerosis. Eur. Neurol. 2002;47(1):11–14. doi: 10.1159/000047940. [http://dx.doi.org/10.1159/000047940]. [PMID: 11803186]. [DOI] [PubMed] [Google Scholar]
- 40.Cinar B.P., Kösehasanoğulları G., Yigit P., Ozakbas S. Cognitive dysfunction in patients with multiple sclerosis treated with first-line disease-modifying therapy: a multi-center, controlled study using the BICAMS battery. Neurol. Sci. 2017;38(2):337–342. doi: 10.1007/s10072-016-2775-7. [http://dx.doi.org/10.1007/s10072-016-2775-7]. [PMID: 27885448]. [DOI] [PubMed] [Google Scholar]
- 41.Butzkueven H., Kappos L., Pellegrini F., Trojano M., Wiendl H., Patel R.N., Zhang A., Hotermans C., Belachew S. Efficacy and safety of natalizumab in multiple sclerosis: interim observational programme results. J. Neurol. Neurosurg. Psychiatry. 2014;85(11):1190–1197. doi: 10.1136/jnnp-2013-306936. [http://dx.doi.org/10.1136/jnnp-2013-306936]. [PMID: 24532785]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mattioli F., Stampatori C., Bellomi F., Scarpazza C., Capra R. Natalizumab significantly improves cognitive impairment over three years in MS: pattern of disability progression and preliminary MRI findings. PLoS One. 2015;10(7):e0131803. doi: 10.1371/journal.pone.0131803. [http://dx.doi. org/10.1371/journal.pone.0131803]. [PMID: 26148120]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kunkel A., Fischer M., Faiss J., Dahne D., Kohler W., Faiss J.H. Impact of natalizumab treatment on fatigue, mood, and as-pects of cognition in relapsing – remitting multiple sclerosis. Front. Neurol. 2015;11(6):97. doi: 10.3389/fneur.2015.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y., Qiu J., Wang Z., You W., Wu L., Ji C., Chen G. Dimethylfumarate alleviates early brain injury and secondary cognitive deficits after experimental subarachnoid hemorrhage via activation of Keap1-Nrf2-ARE system. J. Neurosurg. 2015;123(4):915–923. doi: 10.3171/2014.11.JNS132348. [http://dx.doi.org/10.3171/2014.11.JNS132348]. [PMID: 25614941]. [DOI] [PubMed] [Google Scholar]
- 45.Mohr D.C., Goodkin D.E., Likosky W., Gatto N., Baumann K.A., Rudick R.A. Treatment of depression improves adherence to interferon beta-1b therapy for multiple sclerosis. Arch. Neurol. 1997;54(5):531–533. doi: 10.1001/archneur.1997.00550170015009. [http://dx.doi.org/10.1001/archneur.1997. 00550170015009]. [PMID: 9152109]. [DOI] [PubMed] [Google Scholar]
- 46.Amato M.P., Langdon D., Montalban X., Benedict R.H., DeLuca J., Krupp L.B., Thompson A.J., Comi G. Treatment of cognitive impairment in multiple sclerosis: position paper. J. Neurol. 2013;260(6):1452–1468. doi: 10.1007/s00415-012-6678-0. [http://dx.doi.org/10.1007/s00415-012-6678-0]. [PMID: 23180174]. [DOI] [PubMed] [Google Scholar]
- 47.Sahakian B.J., Morein-Zamir S. Pharmacological cognitive enhancement: treatment of neuropsychiatric disorders and lifestyle use by healthy people. Lancet Psychiatry. 2015;2(4):357–362. doi: 10.1016/S2215-0366(15)00004-8. [http:// dx.doi.org/10.1016/S2215-0366(15)00004-8]. [PMID: 26360089]. [DOI] [PubMed] [Google Scholar]
- 48.Krupp L.B., Christodoulou C., Melville P., Scherl W.F., MacAllister W.S., Elkins L.E. Donepezil improved memory in multiple sclerosis in a randomized clinical trial. Neurology. 2004;63(9):1579–1585. doi: 10.1212/01.wnl.0000142989.09633.5a. [http://dx.doi.org/10.1212/01.WNL.0000142989.09633. 5A]. [PMID: 15534239]. [DOI] [PubMed] [Google Scholar]
- 49.Christodoulou C., MacAllister W.S., McLinskey N.A., Krupp L.B. Treatment of cognitive impairment in multiple sclerosis: is the use of acetylcholinesterase inhibitors a viable option? CNS Drugs. 2008;22(2):87–97. doi: 10.2165/00023210-200822020-00001. [http://dx.doi.org/10.2165/00023210-200822020-00001]. [PMID: 18193921]. [DOI] [PubMed] [Google Scholar]
- 50.Krupp L.B., Christodoulou C., Melville P., Scherl W.F., Pai L.Y., Muenz L.R., He D., Benedict R.H., Goodman A., Rizvi S., Schwid S.R., Weinstock-Guttman B., Westervelt H.J., Wishart H. Multicenter randomized clinical trial of donepezil for memory impairment in multiple sclerosis. Neurology. 2011;76(17):1500–1507. doi: 10.1212/WNL.0b013e318218107a. [http://dx.doi.org/10.1212/WNL.0b013e318218107a]. [PMID: 21519001]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Villoslada P., Arrondo G., Sepulcre J., Alegre M., Artieda J. Memantine induces reversible neurologic impairment in patients with MS. Neurology. 2009;72(19):1630–1633. doi: 10.1212/01.wnl.0000342388.73185.80. [http://dx.doi.org/ 10.1212/01.wnl.0000342388.73185.80]. [PMID: 19092106]. [DOI] [PubMed] [Google Scholar]
- 52.Lovera J.F., Frohman E., Brown T.R., Bandari D., Nguyen L., Yadav V., Stuve O., Karman J., Bogardus K., Heimburger G., Cua L., Remingon G., Fowler J., Monahan T., Kilcup S., Courtney Y., McAleenan J., Butler K., Wild K., Whitham R., Bourdette D. Memantine for cognitive impairment in multiple sclerosis: a randomized placebo-controlled trial. Mult. Scler. 2010;16(6):715–723. doi: 10.1177/1352458510367662. [http://dx.doi.org/10.1177/1352458510367662]. [PMID: 20483885]. [DOI] [PubMed] [Google Scholar]
- 53.Bensa C., Bodiguel E., Brassat D., Laplaud D., Magy L., Ouallet J.C., Zephir H., De Seze J., Blanc F. Multiple Sclerosis Think Tank (Groupe de reflexion sur la sclerose en plaques GRESEP). Recommendations for the detection and therapeutic management of cognitive impairment in multiplesclerosis. Rev. Neurol. (Paris) 2012;168:785–794. doi: 10.1016/j.neurol.2012.02.009. [http://dx.doi.org/10.1016/j.neurol.2012.02. 009]. [PMID: 22658753]. [DOI] [PubMed] [Google Scholar]
- 54.Sachdeva A., Kumar K., Anand K.S. Non-pharmacological cognitive enhancers – current perspectives. J. Clin. Diagn. Res. 2015;9(7):VE01–VE06. doi: 10.7860/JCDR/2015/13392.6186. [PMID: 26393186]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giesser B.S. Exercise in the management of persons with multiple sclerosis. Ther. Adv. Neurol. Disorder. 2015;8(3):123–130. doi: 10.1177/1756285615576663. [http://dx.doi.org/10.1177/1756285615576663]. [PMID: 25941539]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baker L.D., Frank L.L., Foster-Schubert K., Green P.S., Wilkinson C.W., McTiernan A., Plymate S.R., Fishel M.A., Watson G.S., Cholerton B.A., Duncan G.E., Mehta P.D., Craft S. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch. Neurol. 2010;67(1):71–79. doi: 10.1001/archneurol.2009.307. [http://dx.doi.org/10.1001/ archneurol.2009.307]. [PMID: 20065132]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ten Brinke L.F., Bolandzadeh N., Nagamatsu L.S., Hsu C.L., Davis J.C., Miran-Khan K., Liu-Ambrose T. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomised controlled trial. Br. J. Sports Med. 2015;49(4):248–254. doi: 10.1136/bjsports-2013-093184. [http://dx.doi.org/10.1136/ bjsports-2013-093184]. [PMID: 24711660]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hanssen K.T., Beiske A.G., Landrø N.I., Hofoss D., Hessen E. Cognitive rehabilitation in multiple sclerosis: a randomized controlled trial. Acta Neurol. Scand. 2016;133(1):30–40. doi: 10.1111/ane.12420. [http://dx. doi.org/10.1111/ane.12420]. [PMID: 25952561]. [DOI] [PubMed] [Google Scholar]
- 59.Rosti-Otajärvi E.M., Hämäläinen P.I. Neuropsychological rehabilitation for multiple sclerosis. Cochrane Database Syst. Rev. 2014;2(2):CD009131. doi: 10.1002/14651858.CD009131.pub3. [PMID: 24515630]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller E., Kostka J., Włodarczyk T., Dugué B. Whole-body cryostimulation (cryotherapy) provides benefits for fatigue and functional status in multiple sclerosis patients. A case-control study. Acta Neurol. Scand. 2016;134(6):420–426. doi: 10.1111/ane.12557. [http://dx.doi. org/10.1111/ane.12557]. [PMID: 26778452]. [DOI] [PubMed] [Google Scholar]
- 61.McKinley R.A., Bridges N., Walters C.M., Nelson J. Modulating the brain at work using noninvasive transcranial stimulation. Neuroimage. 2012;59(1):129–137. doi: 10.1016/j.neuroimage.2011.07.075. [http://dx.doi.org/10.1016/ j.neuroimage.2011.07.075]. [PMID: 21840408]. [DOI] [PubMed] [Google Scholar]
- 62.Suthana N., Haneef Z., Stern J., Mukamel R., Behnke E., Knowlton B., Fried I. Memory enhancement and deep-brain stimulation of the entorhinal area. N. Engl. J. Med. 2012;366(6):502–510. doi: 10.1056/NEJMoa1107212. [http://dx.doi.org/10.1056/NEJMoa1107212]. [PMID: 22316444]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ross L.A., McCoy D., Wolk D.A., Coslett H.B., Olson I.R. Improved proper name recall by electrical stimulation of the anterior temporal lobes. Neuropsychologia. 2010;48(12):3671–3674. doi: 10.1016/j.neuropsychologia.2010.07.024. [http://dx.doi.org/10.1016/j.neuropsychologia.2010.07.024]. [PMID: 20659489]. [DOI] [PubMed] [Google Scholar]
- 64.Gagnon G., Schneider C., Grondin S., Blanchet S. Enhancement of episodic memory in young and healthy adults: a paired-pulse TMS study on encoding and retrieval performance. Neurosci. Lett. 2011;488(2):138–142. doi: 10.1016/j.neulet.2010.11.016. [http://dx.doi.org/10.1016/j.neulet.2010.11. 016]. [PMID: 21094215]. [DOI] [PubMed] [Google Scholar]
- 65.Teo F., Hoy K.E., Daskalakis Z.J., Fitzgerald P.B. Investigating the role of current strength in tDCS modulation of working memory performance in healthy controls. Front. Psychiatry. 2011;2:45. doi: 10.3389/fpsyt.2011.00045. [http://dx.doi.org/10.3389/fpsyt.2011.00045]. [PMID: 21811474]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.A.; Vahabzadeh, A.M.; Pascue – Leone, A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharm. 2013;64:566–578. doi: 10.1016/j.neuropharm.2012.06.020. [http://dx.doi.org/10.1016/j. neuropharm.2012.06.020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boggio P.S., Fregni F., Bermpohl F., Mansur C.G., Rosa M., Rumi D.O., Barbosa E.R., Odebrecht R.M., Pascual-Leone A., Rigonatti S.P., Marcolin M.A., Araujo S.M.T. Effect of repetitive TMS and fluoxetine on cognitive function in patients with Parkinson’s disease and concurrent depression. Mov. Disord. 2005;20(9):1178–1184. doi: 10.1002/mds.20508. [http://dx.doi.org/10.1002/mds.20508]. [PMID: 15895421]. [DOI] [PubMed] [Google Scholar]
- 68.Holtzheimer P.E., III, McDonald W.M., Mufti M., Kelley M.E., Quinn S., Corso G., Epstein C.M. Accelerated repetitive transcranial magnetic stimulation for treatment-resistant depression. Depress. Anxiety. 2010;27(10):960–963. doi: 10.1002/da.20731. [http://dx.doi.org/10. 1002/da.20731]. [PMID: 20734360]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wishart H.A., Roberts D.W., Roth R.M., McDonald B.C., Coffey D.J., Mamourian A.C., Hartley C., Flashman L.A., Fadul C.E., Saykin A.J. Chronic deep brain stimulation for the treatment of tremor in multiple sclerosis: review and case reports. J. Neurol. Neurosurg. Psychiatry. 2003;74(10):1392–1397. doi: 10.1136/jnnp.74.10.1392. [http://dx.doi.org/ 10.1136/jnnp.74.10.1392]. [PMID: 14570832]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith G.E., Housen P., Yaffe K., Ruff R., Kennison R.F., Mahncke H.W., Zelinski E.M. A cognitive training program based on principles of brain plasticity: results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) study. J. Am. Geriatr. Soc. 2009;57(4):594–603. doi: 10.1111/j.1532-5415.2008.02167.x. [http:// dx.doi.org/10.1111/j.1532-5415.2008.02167.x]. [PMID: 19220558]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller K.J., Dye R.V., Kim J., Jennings J.L., O’Toole E., Wong J., Siddarth P. Effect of a computerized brain exercise program on cognitive performance in older adults. Am. J. Geriatr. Psychiatry. 2013;21(7):655–663. doi: 10.1016/j.jagp.2013.01.077. [http://dx.doi.org/10.1016/j.jagp.2013.01. 077]. [PMID: 23602310]. [DOI] [PubMed] [Google Scholar]
- 72.Whitlock L.A., Collins A.C., Allaire J.C. Individual differences in response to cognitive training: using a multi – modal, attentionally demanding game – based intervention for older adults. Comput. Human Behav. 2012;28:1091–1096. [http://dx.doi.org/10. 1016/j.chb.2012.01.012]. [Google Scholar]
- 73.Anderson C.A., Shibuya A., Ihori N., Swing E.L., Bushman B.J., Sakamoto A., Rothstein H.R., Saleem M. Violent video game effects on aggression, empathy, and prosocial behavior in eastern and western countries: a meta-analytic review. Psychol. Bull. 2010;136(2):151–173. doi: 10.1037/a0018251. [http://dx.doi.org/10.1037/a0018251]. [PMID: 20192553]. [DOI] [PubMed] [Google Scholar]
- 74.Barnes D.E., Yaffe K., Belfor N., Jagust W.J., DeCarli C., Reed B.R., Kramer J.H. Computer-based cognitive training for mild cognitive impairment: results from a pilot randomized, controlled trial. Alzheimer Dis. Assoc. Disord. 2009;23(3):205–210. doi: 10.1097/WAD.0b013e31819c6137. [http://dx.doi.org/10.1097/WAD.0b013e31819c6137]. [PMID: 19812460]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zimmermann R., Gschwandtner U., Benz N., Hatz F., Schindler C., Taub E., Fuhr P. Cognitive training in Parkinson disease: cognition-specific vs nonspecific computer training. Neurology. 2014;82(14):1219–1226. doi: 10.1212/WNL.0000000000000287. [http://dx.doi.org/10.1212/WNL.0000000000000287]. [PMID: 24623840]. [DOI] [PubMed] [Google Scholar]