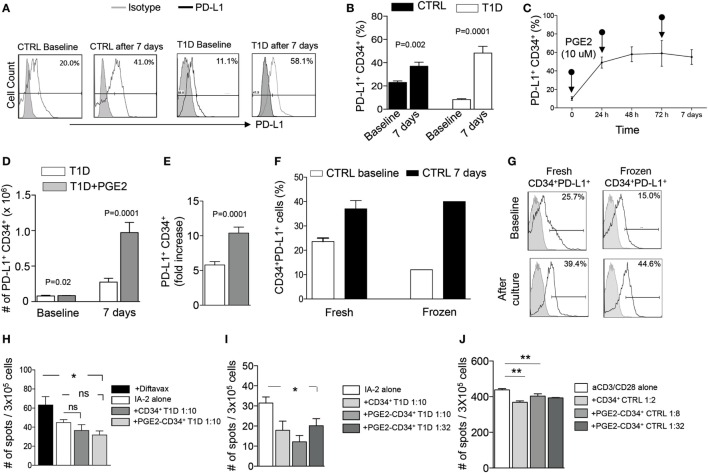
Figure 3.
Effects of human PGE2-modulated and cytokine-treated CD34+ cells. (A,B) Sustained and robust upregulation of PD-L1 upon culture for 7 days with PGE2 and a cocktail of cytokines (heparin, human SCF, human TPO, human FGF-1, IGFBP2, and Angptl3) on CD34+ cells obtained from T1D patients as compared to those from healthy controls. (C) Effect of PGE2 pulsing on CD34+ cells cultured for 0, 24, 72 h and 6 days on PD-L1 expression on CD34+ cells. (D,E) Bar graphs showing an increase in the number of PD-L1+ CD34+ cells and fold increase in cell number after 7 days of culture with PGE2 supplemented with cytokines. (F,G) Upregulation of PD-L1 expression following culture with PGE2 (shown as percentage) on CD34+ cells was not altered by the freeze/thawing process. (H) PGE2-modulated CD34+ cells abrogate the IFN-γ autoimmune response to insulin-associated 2 (I-A2) autoantigen in vitro, as measured via the quantification of IFN-γ-producing cells in an Elispot assay; Diftavax refers to a vaccine including immunization against tetanus toxoid, difteria, and hemophilus. (I) PGE2-modulated CD34+ cells and PGE2-modulated CD34+ cells cultured for 7 days in STFIA media abrogate the IFN-γ autoimmune response toward insulin-associated 2 (I-A2) autoantigen in vitro, as measured via the quantification of IFN-γ-producing cells in an Elispot assay, even when added at low dose. (J) PGE2-modulated CD34+ cells abrogate the anti-CD3/CD28-stimulated PBMC response in vitro as measured via the quantification of IFN-γ-producing cells in an Elispot assay. Data are expressed as mean ± SEM. Data are representative of at least two independent experiments. *P < 0.05; **P < 0.01. Abbreviations: SCF, stem cell factor; TPO, thrombopoietin; hFGF-1, human fibroblast growth factor 1; IGFBP2, insulin-like growth factor-binding protein 2; Angptl3, angiopoietin-like 3; PBMCs, peripheral blood mononuclear cells; PGE2, prostaglandin E2; T1D, type 1 diabetes.