ABSTRACT
The Dps-like peroxide resistance protein (Dpr) is essential for H2O2 stress tolerance and aerobic growth of the oral pathogen Streptococcus mutans. Dpr accumulates during oxidative stress, protecting the cell by sequestering iron ions and thereby preventing the generation of toxic hydroxyl radicals that result from the interaction of iron with H2O2. Previously, we reported that the SpxA1 and SpxA2 regulators positively regulate expression of dpr in S. mutans. Using an antibody raised against S. mutans Dpr, we confirmed at the protein level the central and cooperative nature of SpxA1 and SpxA2 regulation in Dpr production. During phenotypic characterization of the S. mutans Δdpr strain, we observed the appearance of distinct colony variants, which sometimes lost the oxidative stress sensitivity typical of Δdpr strains. Whole-genome sequencing of these phenotypically distinct Δdpr isolates revealed that a putative iron transporter operon, smu995-smu998, was a genomic hot spot with multiple single nucleotide polymorphisms identified within the different isolates. Deletion of smu995 or the entire smu995-smu998 operon in the Δdpr background strain completely reversed the oxidative stress-sensitive phenotypes associated with dpr inactivation. Conversely, inactivation of genes encoding the ferrous iron transport system FeoABC did not alleviate phenotypes of the Δdpr strain. Preliminary characterization of strains lacking smu995-smu998, feoABC, and the iron/manganese transporter gene sloABC revealed the interactive nature of these three systems in iron transport but also indicated that there may be additional iron uptake systems in S. mutans.
IMPORTANCE The dental caries-associated pathogen Streptococcus mutans routinely encounters oxidative stress within the human plaque biofilm. Previous studies revealed that the iron-binding protein Dpr confers protection toward oxidative stress by limiting free iron availability, which is associated with the generation of toxic hydroxyl radicals. Here, we report the identification of spontaneously occurring mutations within Δdpr strains. Several of those mutations were mapped to the operon smu995-smu998, revealing a previously uncharacterized system that appears to be important in iron acquisition. Disruption of the smu995-smu998 operon resulted in reversion of the stress-sensitive phenotype typical of a Δdpr strain. Our data suggest that the Smu995-Smu998 system works along with other known metal transport systems of S. mutans, i.e., FeoABC and SloABC, to coordinate iron uptake.
KEYWORDS: Streptococcus, iron homeostasis, Dpr, oxidative stress
INTRODUCTION
The oral bacterium Streptococcus mutans is a major etiological agent associated with dental carries due to its abilities (i) to synthesize large quantities of extracellular polymers of glucan from sucrose that serve as a scaffold for bacterial tooth colonization, (ii) to transport and metabolize a wide range of carbohydrates at low pH values, and (iii) to survive under acidic pH conditions that are lethal to commensal oral bacteria (1, 2). In dental plaque, S. mutans must also cope with oxidative stresses that arise from bacterial respiration and from the utilization of oral hygiene products (3). In addition, members of the mitis group of streptococci (e.g., S. gordonii and S. sanguinis) are net producers of hydrogen peroxide (H2O2), and a series of studies have demonstrated an inverse correlation between proportions of S. mutans and mitis streptococci in oral health and disease, with high numbers of S. mutans bacteria associated with caries and high proportions of mitis streptococci associated with health (4, 5).
Although H2O2 is a weak oxidizing agent, it is a neutral molecule that quickly penetrates the bacterial membrane and, in the presence of ferrous iron (Fe2+), takes part in the Fenton reaction, which generates highly reactive hydroxyl radicals. In contrast to the case for H2O2, hydroxyl radicals cannot be scavenged by enzymatic reactions and cause irreversible cellular damage by attacking membrane lipids, triggering enzyme mismetallation, or directly targeting proteins and DNA (6). To minimize the deleterious effects of the Fenton reaction, iron uptake in cells is tightly regulated, and any excess of intracellular free iron ions is rapidly immobilized by ubiquitous iron-binding proteins called ferritins (7). In bacteria, ferritin-like proteins are known as Dps (DNA-binding protein from starved cells) or Dpr (Dps-like peroxide resistance), which are functionally similar to but structurally different from mammalian ferritins (8–10). Moreover, some bacterial Dps/Dpr proteins have been shown to bind to DNA, forming a stable complex that further protects the DNA molecule from oxidative damage (11).
In spite of the fact that they do not encode a catalase, streptococci are able to tolerate up to millimolar concentrations of H2O2 (2, 3). In several streptococcal species, Dpr was shown to be essential for bacterial tolerance to oxidative stress (12–16). In S. mutans, the gene encoding Dpr was initially shown to be essential for aerotolerance, as a dpr mutant strain could not grow on agar plates when exposed to air, a phenotype that was fully reversed by the addition of catalase to the growth medium or by anaerobic incubation (14, 17). Recently, we showed that the dpr gene of S. mutans is positively regulated by the oxidative stress regulators SpxA1 and SpxA2 (18). In a recent study that aimed to characterize novel Spx-regulated genes, we included a dpr deletion (Δdpr) strain as a benchmark strain for a number of oxidative stress sensitivity assays (18). In addition to confirming the essentiality of Dpr for aerobic growth and survival in the presence of H2O2, this study also revealed that the Δdpr strain was highly sensitive to iron overload and, most importantly, had reduced infectivity in a rat caries model (18). However, during the study, we noticed the appearance of subpopulations of the Δdpr strain that have lost the heightened sensitivity to oxidative stress. Here, we demonstrate that this phenotypic reversion was stable after multiple passages, and whole-genome sequencing (WGS) of these phenotypically reverted dpr mutant strains revealed single nucleotide polymorphisms (SNPs) in genes coding for a putative Fe3+ ferrichrome ABC transporter (smu995-smu998 operon). By designing clean deletions in this putative iron transport system, we confirmed that disruption of smu995-smu998 reverts the extreme sensitivity of the Δdpr strain to oxidative stress. The present work reveals that the Smu995-Smu998 system is a previously uncharacterized transport system involved in iron uptake and provides novel insights into oxidative stress tolerance mechanisms in S. mutans. Because homologs of Smu995-Smu998 are found in several other streptococci, the findings described here should have broader implications.
RESULTS AND DISCUSSION
Dpr accumulates during aerobic growth and H2O2 stress in an Spx-dependent manner.
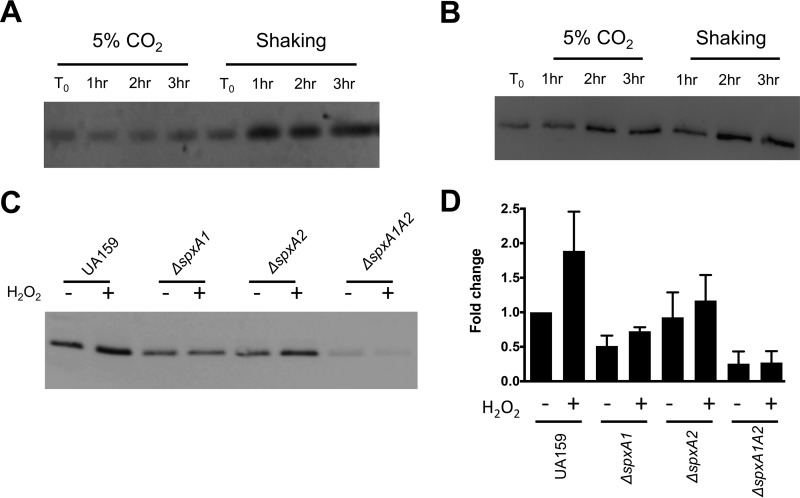
Previously, Yamamoto and colleagues showed that Dpr accumulated in the S. mutans cytoplasm during aerobic growth (9). The high affinity of Dpr for iron and its status as the primary iron-binding protein of streptococci allow the iron-specific stain Ferene-S to be used as a proxy to determine the iron-binding affinity of Dpr (14). To test the impact of exposure to oxygen upon protein-bound iron levels, the S. mutans UA159 and Δdpr strains were grown statically at 37°C in a 5% CO2 incubator or under aeration (shaking culture at 37°C in a standard incubator) for up to 3 h. When protein cell lysates were separated by gel electrophoresis and stained with Ferene-S, a single band of the predicted size of Dpr (∼16 kDa) was detected in the parent strain (Fig. 1A) but not in lysates of the Δdpr strain (data not shown), thereby confirming that the 16-kDa band indeed corresponds to Dpr. The intensity of the staining clearly indicated that more iron molecules were bound to Dpr upon aeration (shaking) than in cells grown statically in 5% CO2 (Fig. 1A). This result suggests that in response to aeration, the abundance of iron molecules bound to Dpr increases as the cells attempt to reduce the availability of free iron that could potentially participate in Fenton chemistry. Based on previous evidence (9, 18), we expected the increase in iron-bound Dpr to correlate with an increase in total Dpr levels. To confirm this possibility, we generated a polyclonal antibody against Dpr and used it to determine Dpr levels in cells grown under the same conditions used for Fig. 1A. As expected, Dpr accumulated over time in cells grown under shaking conditions (Fig. 1B). However, Dpr also accumulated over time in cells grown in 5% CO2, indicating either that some level of oxidative stress occurs in the 5% CO2 atmosphere as the cultures approach stationary phase or that stationary-phase accumulation of Dpr is not oxidative stress dependent. Thus, it appears that the ability of Dpr to sequester iron molecules is enhanced under aeration, supporting data reported when free iron pools were measured before and after aeration in a Δdpr strain of S. mutans GS-5 (9). At this time, it is not completely clear if this phenomenon is due to an increase in the intracellular pools of free iron molecules or to an enhanced efficiency of Dpr to sequester iron during aeration. However, the latter seems more likely, as the 1-h time points reveal similar quantities of Dpr protein in lysates incubated under 5% CO2 or shaking conditions (Fig. 1B), while a notably enhanced potential for iron binding was observed for the shaking cultures (Fig. 1A).
FIG 1.
Accumulation of Dpr during oxidative stress. (A and B) S. mutans UA159 was grown statically in a 5% CO2 atmosphere to mid-log phase (OD600 of 0.4) and split in two aliquots; one was kept in 5% CO2 and the other was incubated under vigorous aeration (shaking at 200 rpm). Protein lysates were prepared from samples that were removed hourly, separated by SDS-PAGE, and subjected to Ferene S staining (20 μg protein lysate per well) (A) or Western blot analysis (2 μg protein lysate per well) (B) using anti-S. mutans Dpr antibodies. (C) Western blot analysis, using anti-S. mutans Dpr antibodies, of lysates from S. mutans UA159 ΔspxA1, ΔspxA2, or ΔspxA1 ΔspxA2 cells (2 μg protein lysate per well) that were grown statically in 5% CO2 to an OD600 of 0.4 and then split in two aliquots, one treated with 0.5 mM H2O2 (+) and one left untreated (−) for 60 min. (D) Densitometry of the Western blots shown in panel C was performed with Bio-Rad ImageQuant software. Data shown are the averages and standard deviations from three independent experiments.
Recently, we showed that transcription of dpr is induced by H2O2 (peroxide) stress and that this induction was dependent on the activity of the transcriptional regulators SpxA1 and SpxA2 (18). In agreement with this observation, Dpr protein levels nearly doubled in the parent strain after exposure to peroxide stress (Fig. 1C). Compared to the parent strain, the ΔspxA1, ΔspxA2, and double mutant ΔspxA1 ΔspxA2 strains harbored reduced basal levels of Dpr. Furthermore, when cultures of the Δspx strains were treated with H2O2, cells lacking SpxA1 but not SpxA2 did not show increased expression of Dpr (Fig. 1C). Notably, production of Dpr was nearly abolished in the ΔspxA1 ΔspxA2 double mutant strain (Fig. 1C). These trends fit well with the transcriptional data previously described, pointing to SpxA1 as the major regulator of dpr expression in response to oxidative stress, while SpxA2 is responsible for basal levels of expression (18). Thus, while dpr may be controlled by other transcriptional factors such as the negative regulator PerR (19), it seems that the bulk of dpr transcription relies on the positive transcriptional activation by SpxA1 and SpxA2.
Phenotypes of the Δdpr strain can be rescued by genetic complementation.
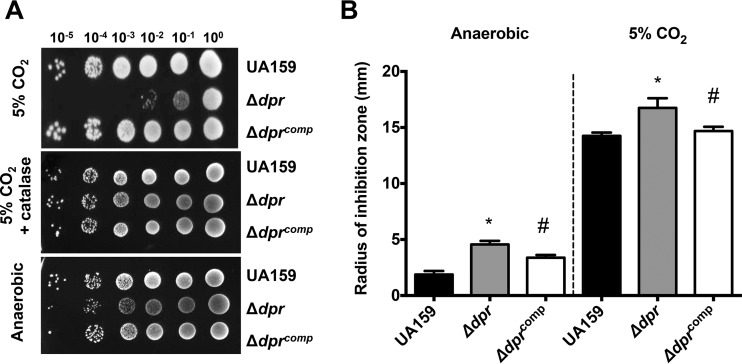
The importance of the S. mutans Dpr in oxidative stress tolerance has been well established, as we and others have shown that inactivation of dpr has multiple negative implications for S. mutans fitness, including extreme sensitivity to growth in air or in the presence of H2O2 (9, 14, 17, 18, 20). As described in greater detail below, the Δdpr strain is phenotypically unstable, a phenomenon first discovered upon observing altered colony morphology of the Δdpr strain struck on agar plates. To ensure that these variants were not the result of unexpected polar effects due to the dpr deletion and because none of the previous studies included the genetic and phenotypic complementation of S. mutans Δdpr strains, we used the integration vector pMC340B to genetically complement the Δdpr strain. As shown before, the Δdpr strain was extremely sensitive to growth in the presence of air (i.e., 5% CO2), a phenotype that could be fully rescued by the addition of catalase to the agar plates or by incubating plates under anaerobic conditions (Fig. 2A). Most importantly, genetic complementation of Δdpr (Δdprcomp strain) fully restored the ability of the strain to grow in the 5% CO2 incubator.
FIG 2.
Genetic complementation of the Δdpr strain. (A) S. mutans UA159, Δdpr, and Δdprcomp strains were grown anaerobically to mid-log phase, at which point 10-fold serial dilutions of cultures were spotted onto BHI agar plates and incubated in a 5% CO2 atmosphere in the presence or absence of catalase or anaerobically. (B) Absence of Dpr increases sensitivity to the iron-dependent antibiotic streptonigrin. Early-log-phase cultures of UA159, Δdpr, and Δdprcomp strains grown anaerobically were swabbed on BHI agar plates and then topped with filter paper discs soaked with a streptonigrin solution. Growth inhibition zones were measured after 24 h of incubation at 37°C, either anaerobically or in 5% CO2. Data shown are the averages and standard deviations for three independent cultures. *, statistical significance compared to UA159 (P < 0.01, one-way analysis of variance [ANOVA]); #, indicates statistical significance compared to the Δdpr strain (P < 0.01, one-way ANOVA).
Sensitivity to the iron-dependent antibiotic streptonigrin has been used as an indirect approach to estimate free iron content in bacterial cells (21). Enhanced sensitivity to streptonigrin indicates that there is a greater abundance of free iron available for coordination with streptonigrin in the cell. As expected, the Δdpr strain was significantly more sensitive than the parent strain to streptonigrin when incubated under either anaerobic or 5% CO2 conditions, a phenotype that was also restored in the Δdprcomp strain (Fig. 2B). Therefore, we can conclude that phenotypic reversions of subpopulations of the Δdpr strain are not associated with polar effects.
Identification of phenotypically unstable populations of the Δdpr strain.
As mentioned above, during phenotypic characterization of the Δdpr strain, we noticed the appearance of two distinct colony morphologies, either “rough” or “smooth,” when the Δdpr strain was struck on agar plates and incubated in a 5% CO2 atmosphere. This observation prompted us to characterize individual Δdpr colony isolates when we observed a bipartite phenotype in individual isolates exposed to a lethal concentration of FeSO4. Specifically, individual colony isolates of the Δdpr strain fell into one of two groups, such that one group (highly sensitive) showed ∼1,000-fold reduction in survival compared to the parent strain, while the other group (moderately sensitive) showed survival rates that were only 10-fold lower than that of the parent strain (Fig. 3). When individual colonies were recovered at the completion of the assay, we found that those Δdpr isolates displaying increased resistance to FeSO4 grew well on plates kept in the CO2 incubator in the absence of catalase and were much less sensitive to H2O2 stress than Δdpr isolates that remained highly sensitive to FeSO4 (data not shown). However, no direct correlation between the colony morphology (i.e., “rough” versus “smooth”) and stress sensitivity could be drawn. Importantly, the phenotype reversions of the Δdpr isolates were stable after multiple passages, suggesting that these Δdpr variants harbor mutations that, at least in part, compensate for the loss of Dpr. As a safeguard, we took measures to avoid the emergence of these variations when working with the Δdpr strain. Specifically, we always worked with fresh plates that were streaked from the frozen stock on plates containing catalase and incubated anaerobically. Once these precautions were adopted, we consistently observed the expected behavior of the Δdpr strain, with high sensitivity to FeSO4 and H2O2 and no noticeable alterations in colony morphology.
FIG 3.
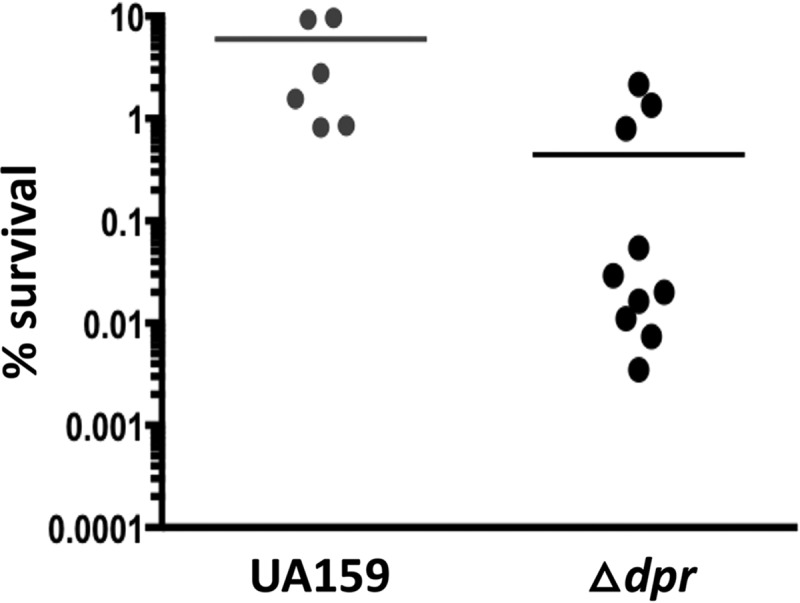
The S. mutans Δdpr strain is phenotypically unstable. When exposed to a lethal concentration of FeSO4 (5 mM), independent cultures of the Δdpr strain fall into two phenotypically distinct clusters: one is highly sensitive (∼3-log-greater sensitivity than UA159), while the other is moderately sensitive to FeSO4.
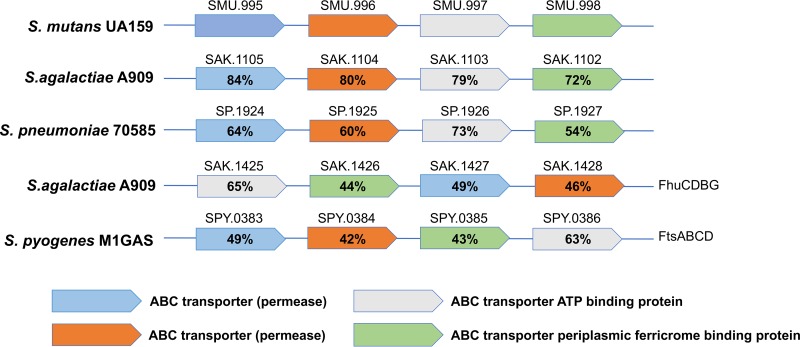
In an attempt to identify a genetic change(s) associated with the phenotypic reversal of a subset of Δdpr isolates, we subjected three Δdpr colonies with different phenotypes to whole-genome sequencing (WGS) analysis: one “smooth” colony that remained stress sensitive (Δdpr-A) and two colonies that had become stress tolerant, one with a “rough” colony phenotype (Δdpr-B) and one with a “smooth” colony phenotype (Δdpr-C). In addition, the lab stock UA159 parent strain was resequenced as a reference to the different Δdpr isolates. Using computational tools (see Materials and Methods) that compared the genome sequences of the three Δdpr isolates to that of our UA159 genome, one or more SNPs were identified in each one of the three Δdpr strains (Table 1). Remarkably, all Δdpr variants displayed SNPs within genes of the smu995-smu998 operon, which is predicted to encode a putative ferric (Fe3+) ferrichrome permease transporter based on BLAST search analysis. The proteins encoded by the smu995-smu998 operon share approximately 80% similarity to a putative ferrichrome permease present in group B Streptococcus (GBS) and in Streptococcus pneumoniae but have limited similarities to other putative ferrichrome permeases previously characterized in group A streptococcus (GAS) and GBS (FtsABCD and FhuCDBG, respectively) (Fig. 4) (22, 23).
TABLE 1.
Spontaneous mutations in Δdpr isolates as determined by whole-genome sequencing
| Isolate (phenotype) | Reference nucleotide (UA159) | Variant nucleotide | Reference amino acid (UA159) | Variant amino acid | Gene locus | Proposed function |
|---|---|---|---|---|---|---|
| Δdpr-A mutant (smooth colony, stress sensitive) | T | A | M | K | smu995 | Iron transport |
| A | T | Intergenic region, smu1378-smu1379 | ||||
| A | T | Intergenic region, smu1378-smu1379 | ||||
| A | G | Intergenic region, smu1378-smu1379 | ||||
| T | A | Intergenic region, smu1378-smu1379 | ||||
| T | G | Intergenic region, smu1635c-smu1636c | ||||
| Δdpr-B mutant (smooth colony, stress tolerant) | T | A | C | S | smu995 | Iron transport |
| G | A | G | S | smu995 | Iron transport | |
| G | T | G | V | smu995 | Iron transport | |
| Δdpr-C mutant (rough colony, stress tolerant) | G | A | R | Q | smu257 (oppC) | ABC transporter |
| G | T | Intergenic region, smu910-smu911c | ||||
| C | A | A | D | smu998 | Iron transport | |
| C | T | A | T | smu2091c (mutS) | DNA repair |
FIG 4.
Similarities of the S. mutans Smu995-Smu998 proteins and homologs in other streptococci as determined by BLAST analysis. Color-coded boxes represent the organization of the genes within various streptococcal species, while the numeric values indicate the percent similarity at the protein level.
In the Δdpr-A isolate, a smooth colony that remained stress sensitive, the annotated start codon of smu995 was changed to a lysine (M→K). Careful observation of the sequence upstream of the putative start codon revealed a second ATG six bases upstream, which was unchanged in the Δdpr-A isolate and may serve as the actual start codon. If this is correct, the SNP that changed the coding sequence from a methionine to a lysine may not have affected Smu995 functionality. Six additional SNPs were identified in the Δdpr-A isolate, although those SNPs were all within noncoding regions. Three SNPs were identified in the Δdpr-B genome (stress-tolerant, rough colony), all within the smu995 coding region, including a cysteine-to-serine substitution (C→S) at residue 66, a glycine to serine substitution (G→S) at residue 67 and a glycine-to-valine substitution (G→V) also at residue 67. While any one of these amino acid substitutions may have potentially affected Smu995 function, the replacement of the cysteine residue by serine is the most likely to impair protein function given that conserved cysteine residues are critical for the function of iron transport proteins of Klebsiella pneumoniae and Pseudomonas aeruginosa (24, 25). Finally, four SNPs were identified in the Δdpr-C isolate (stress-tolerant, smooth colony). Though one substitution was in a noncoding region, the additional SNPs were found within coding regions, including one encoding an arginine-to-glutamine (R→Q) substitution within the gene (smu257) coding for the transmembrane permease OppC, one encoding an alanine-to-threonine (A→T) substitution within the gene (smu2091) coding for the DNA mismatch repair protein MutS, and one encoding an alanine-to-aspartic acid substitution (A→D) within smu998, the last gene of the smu995-smu998 operon. Regardless of whether a particular Δdpr isolate regained oxidative stress tolerance or not, the results from the WGS indicate that the smu995-smu998 operon is a genetic hot spot in the Δdpr strain.
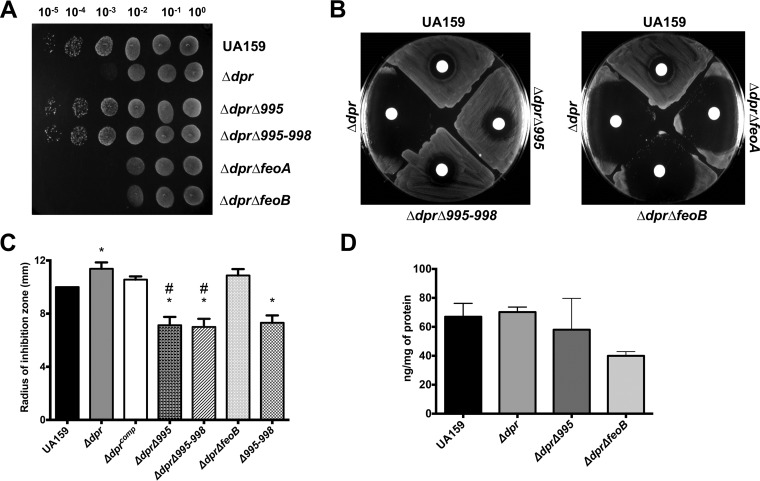
A clean deletion of smu995 or of the entire smu995-smu998 operon alleviates the stress sensitivity of the Δdpr strain.
The multiple SNPs identified within the smu995-smu998 operon in Δdpr variants point to an association between iron uptake by Smu995-Smu998 and Dpr. This association is logical given that in the absence of Dpr, cells might reduce iron uptake to avoid the toxic effects of free iron ions. To assess the role of the Smu995-Smu998 system in alleviation of the Δdpr phenotypes, we used a nonpolar kanamycin resistance cassette to replace the smu995 gene (Δ995) or the entire smu995-smu998 operon (Δ995-998) in both UA159 and Δdpr backgrounds. We also took advantage of the availability of mutant FeoABC ferrous (Fe2+) iron transport system strains of UA159 (ΔfeoA and ΔfeoB) (18) to obtain Δdpr ΔfeoA and Δdpr ΔfeoB double mutant strains. In agreement with predictions based on the WGS results, deletion of smu995 alone or the smu995-smu998 operon in the Δdpr strain restored its ability to grow under aerobic conditions (Fig. 5A) and reversed the high sensitivity of the Δdpr strain to H2O2 in a disc diffusion assay (Fig. 5B). On the other hand, deletion of either feoA or feoB in the Δdpr strain did not affect expression of Δdpr phenotypes (Fig. 5A and B). To estimate the relative availability of free iron within the different strains, we again resorted to the Fe-dependent antibiotic streptonigrin. Inactivation of smu995-smu998 but not of feoB alleviated the increased sensitivity of the Δdpr strain to streptonigrin (Fig. 5C). In fact, the Δdpr Δ995 and Δdpr Δ995-998 double mutant strains were significantly less sensitive to streptonigrin than the parent strain (Fig. 5C), which is consistent with diminished levels of intracellular free iron.
FIG 5.
Inactivation of smu995-smu998 but not of feoAB compensates for the loss of Dpr. (A) Tenfold serial dilutions of mid-exponential-phase cultures of the S. mutans UA159, Δdpr, Δdpr Δsmu995, Δdpr Δsmu995-smu998, Δdpr ΔfeoA, and Δdpr ΔfeoB strains were spotted on BHI agar and incubated in 5% CO2 for 24 h. (B and C) Early-logarithmic-phase cultures of S. mutans strains were swabbed onto a BHI agar plate, upon which filter paper discs saturated with H2O2 (B) or streptonigrin (C) were placed on top of the agar layer. Growth inhibition was observed after 24 h of incubation in 5% CO2. (D) Total iron quantification of mid-logarithmic-phase cultures of S. mutans strains by ICP-OES. Total iron content was normalized to total protein content from the cell lysates. *, statistical significance compared to UA159 (P < 0.05, one-way ANOVA); #, statistical significance compared to the Δdpr strain (P < 0.01, one-way ANOVA).
To determine whether loss of Dpr, either alone or coupled with the Δ995 and ΔfeoB mutations, affected intracellular iron content, we used inductively coupled plasma-optical emission spectrometry (ICP-OES) analyses to quantify the total iron content in the UA159, Δdpr, Δdpr Δ995, and Δdpr ΔfeoB strains grown under 5% CO2 conditions. The total iron contents of the UA159 and Δdpr strains were nearly identical, whereas both the Δdpr Δ995 and Δdpr ΔfeoB strains trended toward reductions in iron content compared to that of the parent and Δdpr strains, though the differences were not significant (Fig. 5D). Interestingly, the transcriptional regulator Spx appears to govern transcription of both the smu995-smu998 and feoABC operons. Recently, we performed transcriptome sequencing (RNA-Seq) analysis to study the H2O2 stress regulon as it relates to Spx regulation in S. mutans (26). When exposed to a subinhibitory concentration of H2O2, transcription of the smu995-smu998 operon was higher in ΔspxA1 and ΔspxA1 ΔspxA2 mutant strains than in the parent strain. During the stress exposure, the expression levels of feoA and feoB mirrored this trend, with increased expression in the Δspx mutant strains compared to UA159. These gene expression trends indicate that Spx proteins serve as important regulators of iron transport systems in S. mutans.
Characterization of strains lacking smu995-smu998, feoABC, and sloABC reveals that the three systems contribute to iron uptake in S. mutans.
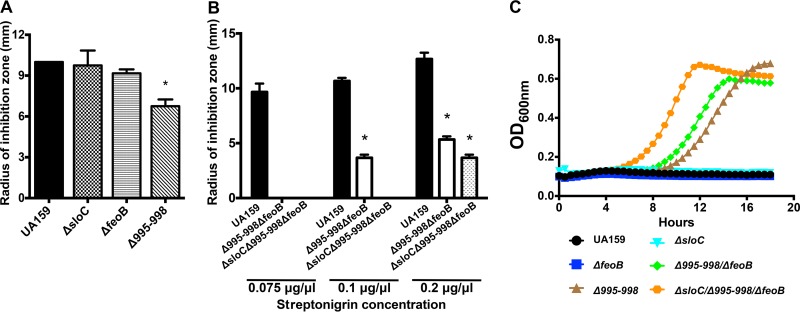
The WGS analysis of the Δdpr revertants and phenotypic characterizations of double Δdpr mutant strains (Δdpr Δ995, Δdpr Δ995-998, Δdpr ΔfeoA, and Δdpr ΔfeoB) suggested that the Smu995-Smu998 operon may be the major iron acquisition system of S. mutans. However, quantification of the total iron content of double mutant strains indicated that the FeoABC system may be more relevant to iron acquisition than Smu995-Smu998 (Fig. 5D). However, in this case, the Δdpr background may be a confounding factor, as the Smu995-Smu998 and FeoABC systems could be responding differently to increases in intracellular free iron. In an attempt to identify the primary transport system responsible for iron uptake, we subjected strains lacking smu995-smu998 (Δ995-998) or feoABC (ΔfeoB) to streptonigrin sensitivity and ICP-OES analyses. We also included a strain lacking the sloC gene (ΔsloC) of the sloABC operon that was available in the lab as part of another project. Although SloABC, an ABC-type transporter, has been characterized as the major manganese transporter of S. mutans, this system is also known to participate in iron transport (27). In addition, we generated a double mutant strain lacking both smu995-smu998 and feoABC (Δ995-998 ΔfeoB) as well as a triple mutant strain lacking all three transporters (ΔsloC Δ995-998 ΔfeoB). Among the single mutant strains, only the Δ995-998 strain showed a small but significant increase in tolerance to streptonigrin in a disc diffusion assay (Fig. 6A) (P < 0.05), which agrees well with the results obtained with the Δdpr double mutants (Fig. 5C). Most importantly, the double (Δ995 ΔfeoB) and triple (ΔsloC Δ995 ΔfeoB) mutant strains showed very high tolerance to streptonigrin, initially demonstrating no detectable growth inhibition at the concentration (0.05 μg μl−1) routinely used to inhibit growth of the parent strain (Fig. 6B). By increasing the concentration of streptonigrin to which the strains were exposed, we were able to obtain the first evidence that the three systems are involved in iron transport, with the double mutant showing some sensitivity at 0.1 μg μl−1 and the triple mutant showing growth inhibition only at the highest concentration (0.2 μg μl−1) of streptonigrin tested (Fig. 6B). Next, we tested the abilities of the different iron transport mutant strains to grow in brain heart infusion (BHI) medium supplemented with 1 μg ml−1 streptonigrin. We observed that disruption of iron transport genes contributed to an enhanced ability to grow in the presence of streptonigrin, with the ΔsloC Δ995-998 ΔfeoB triple mutant strain demonstrating the greatest resistance to streptonigrin, followed by the Δ995-998 ΔfeoB double and Δ995-998 single mutant strains (Fig. 6C). To further demonstrate the importance of iron in streptonigrin sensitivity, we also tested the ability of the Δ995-998 and ΔsloC Δ995-998 ΔfeoB mutant strains to grow in the presence of streptonigrin in broth using the chemically defined medium FMC, replete with or depleted of iron. While growth in iron-replete medium showed that the parent strain was incrementally more sensitive to increased doses of streptonigrin, the strain became completely resistant to high concentrations of streptonigrin when iron was depleted from the growth medium (see Fig. S2 in the supplemental material). As observed in BHI plates, the Δ995-998 single mutant strain and the ΔsloC Δ995-998 ΔfeoB triple mutant strain were highly tolerant to streptonigrin regardless of the iron concentration present in the growth medium (Fig. S2).
FIG 6.
Characterization of the tolerance of S. mutans to the iron-dependent antibiotic streptonigrin following disruption of iron transport systems. (A and B) Measurement of zones of growth inhibition of early-logarithmic-phase cultures of the S. mutans UA159, ΔsloC, ΔfeoB, and Δsmu995-smu998 strains (A) and the UA159, Δ995-998 ΔfeoB, and ΔsloC Δ995-998 ΔfeoB strains (B) incubated in the presence of filter paper discs soaked with streptonigrin for 24 h. A lack of bars in panel B indicates that no growth inhibition was observed. (C) S. mutans UA159 and the panel of iron transport mutant strains were grown in BHI medium supplemented with 1 μg ml−1 of streptonigrin. Cells were grown to an OD600 of 0.5 in BHI and then diluted 1:40 into BHI containing streptonigrin. Growth was monitored using a BioScreen growth reader. *, statistical significance compared to UA159 (P < 0.05, one-way ANOVA).
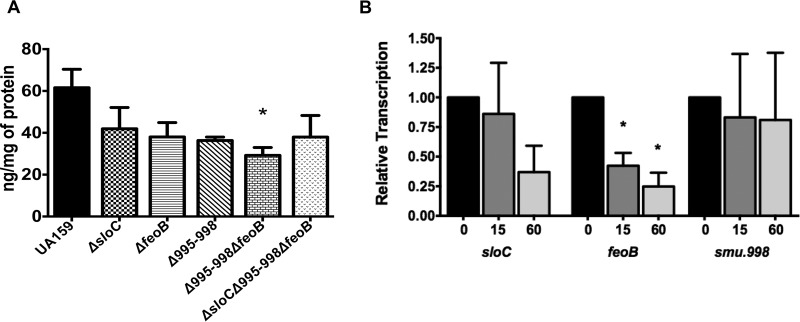
Next, we used ICP-OES to quantify the total iron content in the different iron transport mutants. Interestingly, all single mutant strains showed an approximately 30% reduction in total iron content, further indicating that the three transporters participate in iron acquisition (Fig. 7A). Unexpectedly, the iron contents of both double and triple mutant strains were similar to the iron content found in the single mutant strains, suggesting that S. mutans might have additional iron uptake mechanisms. To shed additional light onto the specific role of each transport system, we used real-time quantitative PCR (qRT-PCR) to explore the transcriptional responses of feoB, sloC, and smu998 after cells of the parent strain were shifted from an anaerobic to an aerobic (via shaking growth) environment. A shift from anaerobic growth to vigorous shaking resulted in diminished relative transcription levels for sloC and feoB but not for smu998 (Fig. 7B).
FIG 7.
Characterization of total iron content and of expression levels of iron transport genes in response to stress. (A) The total cellular iron content of BHI-grown, mid-exponential-phase cultures of S. mutans UA159 and of strains bearing mutations in iron transport genes was determined by ICP-OES analysis. (B) Transcriptional profile of iron transport genes in S. mutans UA159. Cultures were grown in an anaerobic atmosphere to an OD600 of 0.4 (T0) and then exposed to shaking (200 rpm) for 15 or 60 min. Bars represent the relative copy number detected for each gene compared to that at T0. *, statistical significance compared to UA159 (A) or to T0 for each gene (B) (P < 0.05, one-way ANOVA).
Concluding remarks.
In the present study, we showed that the S. mutans Dpr accumulates during oxidative stress in an Spx-dependent manner, and we highlighted the central role of Dpr in oxidative stress and iron homeostasis. Although iron is an essential micronutrient, its involvement in Fenton chemistry and resulting production of highly damaging hydroxyl radicals means that the presence of intracellular free iron must be carefully controlled. Thus, without Dpr, the cell is presented with a unique challenge whereby it must acquire iron from the external environment in order to support enzymatic function, while simultaneously avoiding any excess in iron that, if not chelated by Dpr, can rapidly undergo Fenton chemistry. A common alternative used by Gram-positive bacteria such as S. mutans to avoid iron toxicity is to reduce their intracellular iron content (by either slowing import or increasing export) while bringing in millimolar concentrations of manganese that can be used to replace iron as an enzymatic cofactor (28). While the genome sequencing of Δdpr revertants and subsequent inactivation of the Smu995-Smu998 system confirmed the power of next-generation sequencing to uncover genetic interactions by revealing the intimate linkage of Dpr with Smu995-Smu998, our initial characterization of the iron transport mutants only opens the door for a more thorough characterization of the mechanisms coordinating iron acquisition in S. mutans. Taken together, our results indicate that the smu995-smu998 operon encodes a novel iron transport system of S. mutans that, along with FeoABC and SloABC, participates in iron acquisition. One common mechanism used by bacteria to acquire iron is via production of siderophores, low-molecular-weight organic molecules that are secreted to chelate iron from the environment and are brought back into the cells using surface-associated siderophore-iron receptors. Streptococci, however, are not known to synthesize siderophores (29), and a survey of the S. mutans genome does not reveal the presence of siderophore-encoding genes (27, 30). Interestingly, both GAS and GBS carry siderophore-iron transport genes that appear to mediate transport of siderophores produced by other bacteria, a mechanism known as siderophore piracy (22, 23). Genes of the smu995-smu998 operon share low levels of homology with the siderophore-iron transport genes of GAS and GBS, and the in vitro characterization of strains lacking smu995 or the entire smu995-smu998 operon suggests that the Smu995-Smu998 system plays a role in iron transport even in the absence of siderophores. Previously, it had been proposed that iron import in S. mutans occurs mainly via the transport of Fe2+ iron, rather than Fe3+ iron, which is poorly soluble (30). At this point, it is unclear whether the Smu995-Smu998 system is involved in transporting Fe2+ or Fe3+ ions or whether it mediates iron uptake via siderophores. Finally, total iron quantifications of a triple mutant strain lacking the FeoABC, SloABC, and Smu995-Smu998 transporters strongly indicates that S. mutans possesses an additional, yet-to-be-identified iron transporter(s). Given the importance of iron for bacterial virulence, studies are in progress to further elucidate the mechanisms utilized by S. mutans to acquire and maintain iron homeostasis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 2. The wild-type S. mutans UA159 strain and its derivatives were routinely grown in brain heart infusion (BHI) medium at 37°C in a 5% CO2 atmosphere or under anaerobic conditions. All phenotypic characterization of strains lacking the dpr gene used cultures obtained from a freshly plated frozen stock of the Δdpr strain grown under anaerobiosis (or on plates supplemented with catalase in a 5% CO2 atmosphere) that consistently gave rise only to colonies that maintained high levels of sensitivity to growth in air or in the presence of H2O2. To assess the ability of S. mutans strains to grow in BHI or chemically defined FMC medium, BHI overnight cultures were diluted 1:20 into the appropriate medium and allowed to reach mid-exponential growth phase (optical density at 600 nm [OD600] = 0.5). The cultures were then diluted 1:100 into fresh BHI or FMC medium in a microplate topped with a sterile mineral oil overlay to protect against excess oxidative stress. Growth at 37°C was monitored using the BioScreen growth reader (Oy Growth Curves). Escherichia coli strains were grown in Luria broth at 37°C for plasmid construction and propagation. When required for selective growth of strains, antibiotics were added to the growth medium as appropriate: kanamycin (1,000 μg ml−1 for S. mutans or 100 μg ml−1 for E. coli) or ampicillin (100 μg ml−1 for E. coli).
TABLE 2.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics or application | Source or reference |
|---|---|---|
| Strains | ||
| S. mutans | ||
| UA159 | Wild type | Laboratory stock |
| Δdpr mutant | dpr::Erm | 18 |
| Δdprcomp mutant | Complementation of dpr | This study |
| ΔspxA1 mutant | spxA1::Spec | 35 |
| ΔspxA2 mutant | spxA2::Erm | 35 |
| ΔspxA1 ΔspxA2 mutant | spxA1::Spec spxA2::Erm | 35 |
| Δdpr ΔfeoA mutant | dpr::Erm feoA::Kan | This study |
| Δdpr ΔfeoB mutant | dpr::Erm feoB::Kan | This study |
| Δdpr Δ995 mutant | dpr::Erm smu995::Kan | This study |
| Δdpr Δ995-998 mutant | dpr::Erm smu995-998::Kan | This study |
| Δ995-998 mutant | smu995-998::Kan | This study |
| ΔfeoB mutant | feoB::Kan | 18 |
| ΔsloC mutant | sloC::Spec | This study |
| Δfeo BΔ995-998 mutant | feoB::markerless 995-998::Kan | This study |
| ΔsloC ΔfeoB Δ995-998 mutant | sloC::Spec feoB::markerless 995-998::Kan | This study |
| E. coli | ||
| DH10B | Cloning host | Laboratory stock |
| BL21(λDE3) | Protein expression host | Laboratory stock |
| Plasmids | ||
| pMC340B | S. mutans integration vector | 32 |
| pET21-a(+) | His-tagged protein expression | Millipore Sigma |
To determine Dpr levels under different growth conditions, S. mutans UA159 and Δspx strains were grown in 5% CO2 to an OD600 of 0.4 (T0), and the culture was split in three parts, with one aliquot remaining in the 5% CO2 incubator, another aliquot transferred to a 37°C shaker set to 200 rpm, and the third aliquot kept in 5% CO2 but treated with 0.5 mM H2O2 for selected periods of time. To determine transcription levels of selected iron transport genes, S. mutans UA159 was grown anaerobically in BHI to an OD600 of 0.4. The culture was split in three parts; one was harvested immediately (T0), and the other two were transferred to a 37°C shaker set to 200 rpm for harvesting after 15 or 60 min. Cells were harvested by centrifugation, resuspended in 1 ml RNAprotect bacterial reagent (Qiagen), and incubated at room temperature for 5 min. After an additional round of centrifugation, the supernatant was discarded, and cell pellets were stored at −80°C until use.
Construction of mutant and complemented strains.
The S. mutans strains lacking the smu995 or sloC gene or the entire smu995-smu998 operon were constructed using a PCR ligation mutagenesis approach (31). Briefly, PCR fragments flanking the desired region to be deleted were ligated to a nonpolar kanamycin cassette (NP-Kmr) and the ligation mix used to transform S. mutans UA159. Double mutants were obtained by amplifying the mutated dpr region of the previously constructed Δdpr strain (18) and then using this PCR product to transform the newly generated single mutant strains or the already available ΔfeoB strain (18). Mutant strains were isolated on BHI plates supplemented with the appropriate antibiotic(s), and the deletions were confirmed by PCR sequencing of the insertion site and flanking region. Complementation of the Δdpr strain was achieved by cloning the dpr gene into the S. mutans integration vector pMC340B (32). Briefly, the dpr coding sequence was amplified by PCR and cloned into pMC340B to yield plasmid pMC340B-dpr. The plasmid was isolated in E. coli DH10B and used to transform the S. mutans Δdpr strain for integration at the mtl locus. Primers used in this study are listed in Table 3.
TABLE 3.
Primers used in this study
| Primer | Sequence (5′→3′)a | Application |
|---|---|---|
| dpr comp 5′KpnI | TTCGTTAAAGGGTACCAAATAATTGCT | dpr complementation |
| dpr comp 3′KpnI | GACTTCCTATCTGGTACCACTTATAAACC | |
| Dpr 5′NdeI | GAATATTTTTTAAGGAGGAATTCATATGACGAATAC | Dpr overexpression in E. coli |
| Dpr 3′XhoI | CTATCTTCACACTCGAGTAAACCGGGAG | |
| dpr Arm1 Forward | CAATCAGCCAGTCGGG | Construction of double Δdpr mutants |
| dpr Arm2 Reverse | GCTGAAGCCACTGTTTC | |
| sloC 5′Arm1 | GATCACGTTCTGCTTTTG | sloC deletion |
| sloC 3′Arm1 SphI | GTAATAATAAGCTTAGCATGCTCATTAG | |
| sloC 5′Arm2 SphI | GGTTGTTTCGCATGCTTCTCTTAAG | |
| sloC 3′Arm2 | GATGCTGTTCCATATAC | |
| 995 5′Arm1 | CGAACCATGATTGTTGGTATTCCC | smu995 deletion |
| 995 3′Arm1 BamHI | CCAGCACTGGATCCGTGAGTAC | |
| 995 5′Arm2 BamHI | CTATAGCTGGATCCGGAGAAG | |
| 995 3′Arm2 | CGCCACTTAATTCATCAAGAAAGCG | |
| 995-998 5′Arm1 | CGAACCATGATTGTTGGTATTCCC | smu995-smu998 operon deletion |
| 995-998 3′Arm1 BamHI | CCAGCACTGGATCCGTGAGTAC | |
| 995-998 5′Arm2 BamHI | GGTAAAAATTGGATCCTTAATACAGGTGG | |
| 995-998 3′Arm2 | GCATTTTGCTCCTTCTTGAATCAGG | |
| sloC RT Fwd | CAGTGAGCGATAGTGTTAAGAC | sloC qRT-PCR |
| sloC RT Rev | CTCTTGTTTTTAGGATCTTTTTCAGATAG | |
| feoB RT Fwd | GGCTTATCAGATGGGAGTGC | feoB qRT-PCR |
| feoB RT Rev | GCAATCGCTGCTTCAAATTTGG | |
| smu998 RT Fwd | GAGCAACAATTGGTCAGGATGC | smu998 qRT-PCR |
| smu998 RT Rev | GCTTTGGTTGTTCTAATACCACCTG |
Underlining indicates restriction endonuclease recognition sites.
Stress survival assays.
To test sensitivity to H2O2 (Sigma-Aldrich) or streptonigrin (Sigma-Aldrich) in disc diffusion assays, a uniform layer of exponentially grown cells was spread onto agar plates using a sterile swab. Then, Whatman filter paper discs (6-mm diameter) saturated with 20 μl of 0.5% H2O2 solution or 0.05 μg μl−1 streptonigrin (unless a different concentration is mentioned) were placed on the agar. The radius of the zone of growth inhibition was measured after 24 h of incubation at 37°C in 5% CO2 or under anaerobic conditions. To test sensitivity to ferrous iron (FeSO4), exponentially grown cells were diluted 1:100 into plain BHI or BHI supplemented with 5 mM FeSO4. The cultures were incubated for 24 h at 37°C in 5% CO2, at which point 10-fold serial dilutions were prepared and plated for CFU enumeration.
WGS.
S. mutans UA159 and three Δdpr colony isolates were selected for whole-genome sequencing (WGS). The Δdpr isolates were chosen to represent all combinations of phenotypes routinely observed for this strain, which included different morphologies (rough or smooth) demonstrating either sensitivity or resistance to oxidative stress. Upon confirmation that the stress phenotype was stable after multiple passages, 3 ml of cultures grown overnight in BHI under 5% CO2 was harvested by centrifugation at 4°C for 10 min at 4,000 rpm, and the chromosomal DNA was isolated using the Qiagen DNeasy blood and tissue kit following the manufacturer's instructions for isolation of Gram-positive bacterial DNA, which included the addition of 25 U mutanolysin and 400 μg RNase A per sample during the pretreatment step. The WGS was performed at the University of Rochester Genomics Research Center (UR-GRC) using the Illumina MiSeq platform. Sequence reads were cleaned using Trimmomatic-0.32 before mapping to the UA159 genome using SHRiMP2.2.3. The genome sequences of the Δdpr isolates were compared to that of UA159 to search for SNPs using SAMtools-1.2 and BCFtools-1.0.
Purification of recombinant Dpr and antibody generation.
To express and purify Dpr, the promoter and coding region of the dpr gene were amplified from S. mutans UA159 using primers listed in Table 3. The amplified fragment and the expression vector pET-21a(+) (Millipore Sigma) were digested with NdeI and XhoI, ligated, and transformed by electroporation into E. coli BL21(λDE3). An isolate harboring the pET21a-rDpr plasmid was grown in LB containing ampicillin to an OD600 of 0.5, and expression of the His-tagged fusion protein was induced by treatment with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 4 h. The recombinant protein was purified using the Ni-nitrilotriacetic acid (NTA) protein purification kit (Qiagen) according to the supplier's instructions. For generation of anti-rDpr polyclonal antibodies, adult rabbits were immunized with 1 mg of rDpr with Freund's adjuvant intravenously at days 1, 21, and 42 at Lampire Biological Laboratories (Pipersville, PA). After 50 days, the animals were bled, the titer of polyclonal antibodies in the serum against Dpr was determined by enzyme-linked immunosorbent assay, and the antibody specificity was confirmed by Western blotting using the wild-type and Δdpr strains (data not shown).
Western blot analysis.
Whole-cell protein lysates of S. mutans UA159, Δdpr, or Δspx strains were obtained by homogenization in the presence of 0.1-mm glass beads using a bead beater (Biospec). For staining of iron-binding proteins, Fe(NH4)2(SO4) was added to the whole-cell protein lysates at a final concentration of 1 mM and incubated on ice for 30 min. The protein extracts were resolved by 8% nondenaturing PAGE (native PAGE) and stained with Ferene-S solution (2% acetic acid containing 1 mM Ferene-S and 15 mM thioglycolic acid) for 30 min at room temperature (33). Duplicate gels were stained with Coomassie brilliant blue as a loading control. To detect Dpr levels in the different S. mutans strains, protein lysates were separated by 15% SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore). Dpr detection was performed using rabbit anti-rDpr polyclonal antibody diluted 1:1,000 in phosphate-buffered saline (PBS) containing 0.1% Tween 20 (PBS-T) and anti-rabbit horseradish peroxidase (HRP)-coupled antibody (Sigma-Aldrich) diluted 1:2,000 in PBS-T. Immune reactivity was visualized by the incubation of the blot with 3,3-diaminobenzidine (Sigma-Aldrich) substrate solution for horseradish peroxidase conjugates. Densitometry was performed with Bio-Rad ImageQuant software in three independent experiments.
ICP-OES analysis.
The total metal content within bacterial cells was determined by inductively coupled plasma-optical emission spectrometry (ICP-OES) at the University of Florida Institute of Food and Agricultural Sciences (UF-IFAS) Analytical Services Laboratories. Briefly, cultures (250 ml) were grown to mid-exponential phase, harvested by centrifugation at 4°C for 15 min at 4,000 rpm, and washed twice in ice-cold PBS supplemented with 0.5 mM EDTA to chelate extracellular divalent cations. Then, bacterial pellets were resuspended in 2 ml 35% HNO3 and digested at 90°C for 1 h in a high-density polyethylene scintillation vial (Fisher Scientific). Digested bacteria were diluted 1:10 in reagent-grade H2O prior to ICP-OES metal analysis. The metal composition was quantified using a 5300DV ICP atomic emission spectrometer (PerkinElmer), and concentrations were determined by comparisons to a standard curve. Metal concentrations were normalized to total protein content determined by the bicinchoninic acid (BCA) assay.
RNA extraction and qRT-PCR.
Total RNA was isolated from S. mutans using our standard protocols as described previously (34). Briefly, RNA was isolated from homogenized S. mutans cells by acid-phenol-chloroform extractions and treated with DNase I (Ambion) at 37°C for 30 min. The RNA was purified using the RNeasy minikit (Qiagen), including two additional on-column DNase treatments as recommended by the supplier. RNA concentrations were determined using a NanoDrop spectrophotometer (Thermo Fisher). For real-time quantitative PCR (qRT-PCR), cDNA templates were created from 1 μg of RNA using MultiScribe reverse transcriptase (Applied Biosystems). Reactions were carried out on a CFX96 RT system (Bio-Rad) using iTaq Universal SYBR green Supermix (Bio-Rad) and the primer sets listed in Table 3.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by NIH-NIDCR RO1 award DE019783 to J.A.L. The authors from the University of Campinas were supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) (6849-12-1) and FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) (2012/032278-3 and 2014/03816-4).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00062-18.
REFERENCES
- 1.Bowen WH, Koo H. 2011. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res 45:69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemos JA, Burne RA. 2008. A model of efficiency: stress tolerance by Streptococcus mutans. Microbiology 154:3247–3255. doi: 10.1099/mic.0.2008/023770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marquis RE. 1995. Oxygen metabolism, oxidative stress and acid-base physiology of dental plaque biofilms. J Ind Microbiol 15:198–207. doi: 10.1007/BF01569826. [DOI] [PubMed] [Google Scholar]
- 4.Giacaman RA, Torres S, Gomez Y, Munoz-Sandoval C, Kreth J. 2015. Correlation of Streptococcus mutans and Streptococcus sanguinis colonization and ex vivo hydrogen peroxide production in carious lesion-free and high caries adults. Arch Oral Biol 60:154–159. doi: 10.1016/j.archoralbio.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Kreth J, Zhang Y, Herzberg MC. 2008. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J Bacteriol 190:4632–4640. doi: 10.1128/JB.00276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imlay JA. 2013. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol 11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison PM, Arosio P. 1996. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta 1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 8.Calhoun LN, Kwon YM. 2011. The ferritin-like protein Dps protects Salmonella enterica serotype Enteritidis from the Fenton-mediated killing mechanism of bactericidal antibiotics. Int J Antimicrob Agents 37:261–265. doi: 10.1016/j.ijantimicag.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto Y, Fukui K, Koujin N, Ohya H, Kimura K, Kamio Y. 2004. Regulation of the intracellular free iron pool by Dpr provides oxygen tolerance to Streptococcus mutans. J Bacteriol 186:5997–6002. doi: 10.1128/JB.186.18.5997-6002.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao G, Ceci P, Ilari A, Giangiacomo L, Laue TM, Chiancone E, Chasteen ND. 2002. Iron and hydrogen peroxide detoxification properties of DNA-binding protein from starved cells. A ferritin-like DNA-binding protein of Escherichia coli. J Biol Chem 277:27689–27696. [DOI] [PubMed] [Google Scholar]
- 11.Martinez A, Kolter R. 1997. Protection of DNA during oxidative stress by the nonspecific DNA-binding protein Dps. J Bacteriol 179:5188–5194. doi: 10.1128/jb.179.16.5188-5194.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hua CZ, Howard A, Malley R, Lu YJ. 2014. Effect of nonheme iron-containing ferritin Dpr in the stress response and virulence of pneumococci. Infect Immun 82:3939–3947. doi: 10.1128/IAI.01829-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsou CC, Chiang-Ni C, Lin YS, Chuang WJ, Lin MT, Liu CC, Wu JJ. 2010. Oxidative stress and metal ions regulate a ferritin-like gene, dpr, in Streptococcus pyogenes. Int J Med Microbiol 300:259–264. doi: 10.1016/j.ijmm.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto Y, Poole LB, Hantgan RR, Kamio Y. 2002. An iron-binding protein, Dpr, from Streptococcus mutans prevents iron-dependent hydroxyl radical formation in vitro. J Bacteriol 184:2931–2939. doi: 10.1128/JB.184.11.2931-2939.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang T, Ding Y, Li T, Wan Y, Li W, Chen H, Zhou R. 2012. A Fur-like protein PerR regulates two oxidative stress response related operons dpr and metQIN in Streptococcus suis. BMC Microbiol 12:85. doi: 10.1186/1471-2180-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu L, Kreth J. 2012. The role of hydrogen peroxide in environmental adaptation of oral microbial communities. Oxid Med Cell Longev 2012:717843. doi: 10.1155/2012/717843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto Y, Higuchi M, Poole LB, Kamio Y. 2000. Role of the dpr product in oxygen tolerance in Streptococcus mutans. J Bacteriol 182:3740–3747. doi: 10.1128/JB.182.13.3740-3747.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galvao LC, Miller JH, Kajfasz JK, Scott-Anne K, Freires IA, Franco GC, Abranches J, Rosalen PL, Lemos JA. 2015. Transcriptional and phenotypic characterization of novel Spx-regulated genes in Streptococcus mutans. PLoS One 10:e0124969. doi: 10.1371/journal.pone.0124969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujishima K, Kawada-Matsuo M, Oogai Y, Tokuda M, Torii M, Komatsuzawa H. 2013. dpr and sod in Streptococcus mutans are involved in coexistence with S. sanguinis, and PerR is associated with resistance to H2O2. Appl Environ Microbiol 79:1436–1443. doi: 10.1128/AEM.03306-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto Y, Higuchi M, Poole LB, Kamio Y. 2000. Identification of a new gene responsible for the oxygen tolerance in aerobic life of Streptococcus mutans. Biosci Biotechnol Biochem 64:1106–1109. doi: 10.1271/bbb.64.1106. [DOI] [PubMed] [Google Scholar]
- 21.Yeowell HN, White JR. 1982. Iron requirement in the bactericidal mechanism of streptonigrin. Antimicrob Agents Chemother 22:961–968. doi: 10.1128/AAC.22.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clancy A, Loar JW, Speziali CD, Oberg M, Heinrichs DE, Rubens CE. 2006. Evidence for siderophore-dependent iron acquisition in group B streptococcus. Mol Microbiol 59:707–721. doi: 10.1111/j.1365-2958.2005.04974.x. [DOI] [PubMed] [Google Scholar]
- 23.Hanks TS, Liu M, McClure MJ, Lei B. 2005. ABC transporter FtsABCD of Streptococcus pyogenes mediates uptake of ferric ferrichrome. BMC Microbiol 5:62. doi: 10.1186/1471-2180-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hung KW, Tsai JY, Juan TH, Hsu YL, Hsiao CD, Huang TH. 2012. Crystal structure of the Klebsiella pneumoniae NFeoB/FeoC complex and roles of FeoC in regulation of Fe2+ transport by the bacterial Feo system. J Bacteriol 194:6518–6526. doi: 10.1128/JB.01228-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seyedmohammad S, Fuentealba NA, Marriott RA, Goetze TA, Edwardson JM, Barrera NP, Venter H. 2016. Structural model of FeoB, the iron transporter from Pseudomonas aeruginosa, predicts a cysteine lined, GTP-gated pore. Biosci Rep 36:e00322. doi: 10.1042/BSR20160046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kajfasz JK, Ganguly T, Hardin EL, Abranches J, Lemos JA. 2017. Transcriptome responses of Streptococcus mutans to peroxide stress: identification of novel antioxidant pathways regulated by Spx. Sci Rep 7:16018. doi: 10.1038/s41598-017-16367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paik S, Brown A, Munro CL, Cornelissen CN, Kitten T. 2003. The sloABCR operon of Streptococcus mutans encodes an Mn and Fe transport system required for endocarditis virulence and its Mn-dependent repressor. J Bacteriol 185:5967–5975. doi: 10.1128/JB.185.20.5967-5975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eijkelkamp BA, McDevitt CA, Kitten T. 2015. Manganese uptake and streptococcal virulence. Biometals 28:491–508. doi: 10.1007/s10534-015-9826-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner AG, Ong CY, Walker MJ, Djoko KY, McEwan AG. 2017. Transition metal homeostasis in Streptococcus pyogenes and Streptococcus pneumoniae. Adv Microb Physiol 70:123–191. doi: 10.1016/bs.ampbs.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Evans SL, Arceneaux JE, Byers BR, Martin ME, Aranha H. 1986. Ferrous iron transport in Streptococcus mutans. J Bacteriol 168:1096–1099. doi: 10.1128/jb.168.3.1096-1099.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau PC, Sung CK, Lee JH, Morrison DA, Cvitkovitch DG. 2002. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J Microbiol Methods 49:193–205. doi: 10.1016/S0167-7012(01)00369-4. [DOI] [PubMed] [Google Scholar]
- 32.Chen PM, Chen YY, Yu SL, Sher S, Lai CH, Chia JS. 2010. Role of GlnR in acid-mediated repression of genes encoding proteins involved in glutamine and glutamate metabolism in Streptococcus mutans. Appl Environ Microbiol 76:2478–2486. doi: 10.1128/AEM.02622-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsou CC, Chiang-Ni C, Lin YS, Chuang WJ, Lin MT, Liu CC, Wu JJ. 2008. An iron-binding protein, Dpr, decreases hydrogen peroxide stress and protects Streptococcus pyogenes against multiple stresses. Infect Immun 76:4038–4045. doi: 10.1128/IAI.00477-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abranches J, Candella MM, Wen ZT, Baker HV, Burne RA. 2006. Different roles of EIIABMan and EIIGlc in regulation of energy metabolism, biofilm development, and competence in Streptococcus mutans. J Bacteriol 188:3748–3756. doi: 10.1128/JB.00169-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kajfasz JK, Martinez AR, Rivera-Ramos I, Abranches J, Koo H, Quivey RG Jr, Lemos JA. 2009. Role of Clp proteins in expression of virulence properties of Streptococcus mutans. J Bacteriol 191:2060–2068. doi: 10.1128/JB.01609-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.