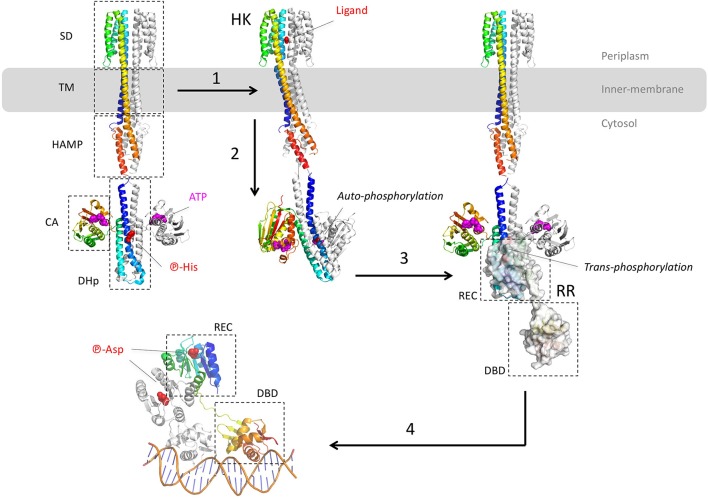
Figure 2.
Structural overview of the two-component systems (TCS). The different model structures of histidine kinases (HK) are from the E. coli NarQ, CpxA, and HK853 (pdb codes 5JEQ, 5IEF, 4BIV, and 2C2A). The model structures of the response regulator (RR) are from the E. coli KdpE and RR468 (pdb codes 3DGE, 4KFC, and 4KNY). The different protein domains are indicated in dashed-squares: Sensor Domain (SD), TransMembrane domain (TM), transduction domain HAMP (histidine kinases, adenyl cyclases, methylaccepting proteins, phosphatases), Catalytic Domain (CA), Dimerisation, and Histidine phosphotransfer domain (DHp), Receiver domain (REC), and Dinucleotidic acid Binding Domain (DBD). (1) After ligand binding to the p-helices (NO3 in the example of NarQ sensor kinase) of the symmetrical receptor homodimer, (2) important helices rearrangement forms an asymmetrical active state which triggers the auto-phosphorylation of DHp by CA. In this example, the CA of one monomer brings the ATP to the DHp of the other monomer. (3) The phosphor-histidine is then exposed to the REC domain of the response regulator (surface representation) that takes over the same phosphate following HK-RR complex formation. (4) Dimerisation of the aspartyl-phosphorylated RR allows DNA recognition by the DBD domain in a tandem manner, with the helix-turn-helix motif inside the major groove and the hairpin winged in the minor groove. All the 3D structures are generated with PyMol (http://www.pymol.org; DeLano, 2009).