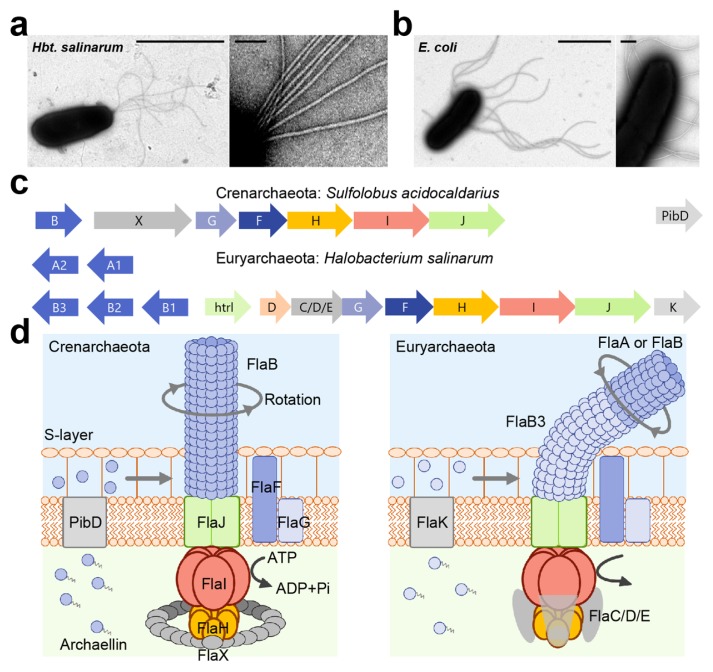
Figure 1.
Motility structure of archaea, archaellum (a) Electron micrograph of Halobacterium salinarum cell (left) and magnified image of archaella (right). Scale bars, 2 μm (left) and 0.1 μm (right). (b) Electron micrograph of Escherichia coli cell (left) and magnified image of flagella (right). Scale bars, 2 μm (left) and 0.2 μm (right). (c) The organization of archaella operon. Homologous genes are represented in the same colors. (d) The current model of archaellar motor. Pre-archaellin has a short signal sequence, and the polymerization proceed after removing this signal sequence by the signal peptidase FlaK/PibD in Euryarchaeota and PibD for Crenarchaeota. In Crenarchaeota, archaellar filament is composed of a single archaellin, FlaB, whereas Euryarchaeota possess the several archaellin genes, flaA or flaB. FlaB3 might play role of hook in Euryarchaeota. Motor is composed of a conserved the membrane protein (FlaJ), the hexametric ATPase (FlaI) and the regulator of ATPase activity (FlaH). The C-terminal domain of FlaI contains the Walker A and B motif for ATP-binding and hydrolysis, and its ATPase activity is essential for not only archaellum assembly and rotation. Nine to ten FlaH molecules assemble in a ring complex and regulates the ATPase activity of FlaI. In addition, Euryarchaeota form FlaC/D/E for motor complex, whereas Crenarchaeota possess FlaX instead. FlaC/D/E play a role for the control of rotational direction by interacting with Che proteins.