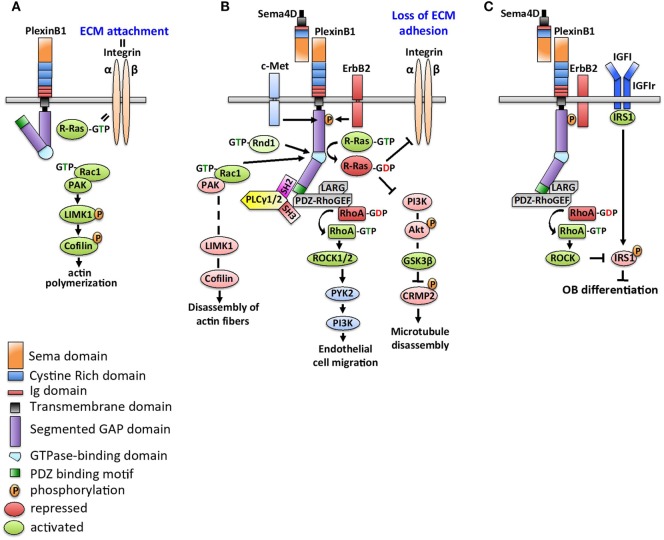
Figure 1.
Schematics of Sema4D/Plexin-B1 signaling. (A) In the absence of Sema4D, the cytoplasmic tail of Plexin-B1 is in an inactive conformation. R-Ras is in a GTP-bound state to collaborate with integrin and control cellular adhesion to the extracellular matrix (ECM). With Plexin-B1 in an inactive state, Rac activates p21-activated kinase (PAK) to trigger LIMK1 signaling and downstream phosphorylation of cofilin, which results in increased actin polymerization and microtubule assembly. (B) Sema4D binding to Plexin-B1 alters the Plexin-B1 conformation, recruits Rac1 to the complex, and inhibits activation of the PAK–LIMK1–cofilin signaling cascade. In addition, R-Ras recruitment to the Sema4D/Plexin-B1 complex inhibits its ability to regulate integrin-mediated activation, resulting in increased cell motility. Deactivation of R-Ras further decreases PI3K-Akt GSK3β pathway, which leads to deactivation of CRMP-2 and subsequent microtubule disassembly. In an ErbB2-dependent signaling step, PLCγ1/2 is recruited to Plexin-B1 via its SH2 domain. Structural interaction of the PLCγ1/2 SH3 domain triggers PDZ-RhoGEF/LARG complex, resulting in RhoA activation. Active GTP-RhoA acts on Raf and Rho-associated kinases (ROCK1/2), which in turn stimulate PYK2 to induce cell invasiveness and migration. (C) Sema4D-Plexin-B1 signaling inhibits osteoblast differentiation. Plexin-B1/ErbB2-dependent RhoA activation stimulates activation of downstream kinase ROCK, which phosphorylates the insulin receptor substrate 1 (IRS1). This causes suppression of insulin like growth factor (IGF1)-dependent signaling and blocks osteoblast differentiation.