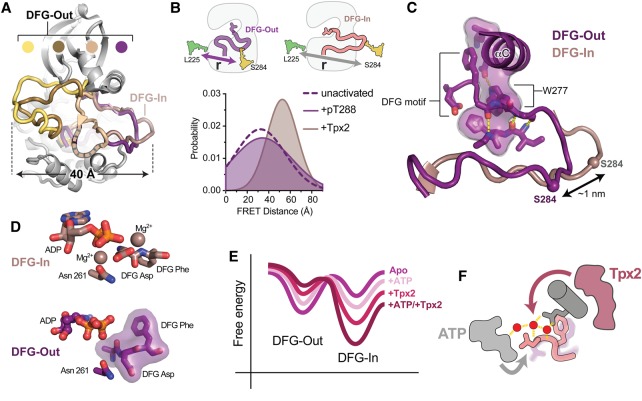
Figure 3. Tpx2 binding to AurA triggers a conformational transition from a DFG-Out state to the active DFG-In state.
(A) Selected crystal structures of AurA in which the kinase adopts DFG-Out states with widely differing positioning of the activation loop [PDB IDs: 3UOK (yellow), 2WTV (brown), 5EW9 (beige) and 5L8K (dark purple)]. The active DFG-In state is highlighted for comparison (pink, PDB ID: 1OL5). Structures were aligned on the entire kinase domain. (B) Schematics of the dye-labeling scheme used to track the position of the activation loop by FRET (top), and (bottom) the distribution of inter-dye distances determined by time-resolved FRET for inactive AurA (dashed line), AurA activated by Tpx2 (pink), and AurA activated by phosphorylation on T288 (dark purple-shaded area). The data are taken from Ruff et al. [88]. (C) Structure of the probable DFG-Out state adopted by AurA in solution (purple). The flipped DFG motif is indicated, and the partially intact regulatory spine in which the W277 residue has taken the place of the DFG phenylalanine residue, is shown as a transparent surface. The conformation of the activation loop in the active DFG-In state is shown for reference (pink). The Cα atom of the residue labeled with a fluorescent dye is shown as a sphere in both structures. (D) Top: View of the active site in the active DFG-In state showing the bound nucleotide and magnesium ions, the DFG motif and the Asn 261 residue, which, together with the DFG Asp, co-ordinates one of the magnesium ions (PDB ID: 1OL5). Bottom: View of the active site in the crystal structure of the native DFG-Out state showing bound nucleotide, the flipped DFG motif and the Asn261 residue (PDB ID 5L8K). Magnesium coordination is lost in this state. (E) Illustrative free energy landscape for the DFG-In/DFG-Out transition, showing how nucleotide and Tpx2 binding lead to graded changes in the populations of the DFG-In and DFG-Out states. (F) Schematic of the AurA active site showing the DFG motif (light pink), αC-helix and Q185 residue (dark gray), water network (red spheres and yellow hydrogen bonds) along with bound Tpx2 (magenta) and ATP (light gray). The colored arrows represent the allosteric coupling of Tpx2 to the DFG motif through the water network (magenta), and the coupling of ATP to the DFG motif through the water network and magnesium ions (gray). The faded DFG-Out state (purple) represents the promotion of the DFG-In state by nucleotide and Tpx2.