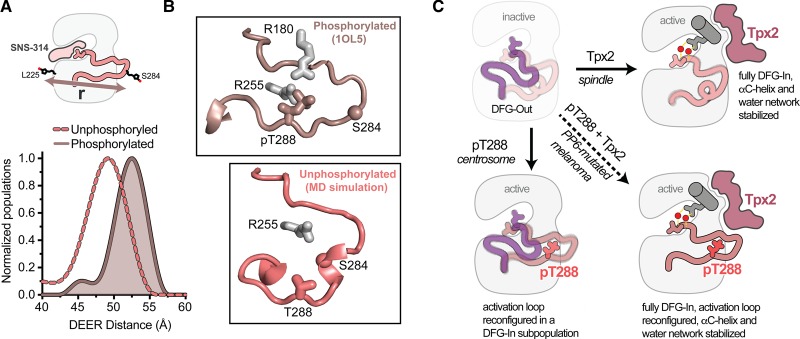
Figure 4. Phosphorylation of T288 triggers a rearrangement of the activation loop within the DFG-In state.
(A) Schematic of the spin-probe labeling scheme (top) and measured spin-spin distances (bottom) from double electron–electron resonance experiments on phosphorylated AurA (darker solid line and shading) and unphosphorylated AurA (lighter dashed line). Data are taken from Ruff et al. [88]. The inhibitor SNS-314 was used to isolate the DFG-In state. (B) Conformation of the activation loop in the active DFG-In substate promoted by phosphorylation on T288 (top, PDB ID: 1OL5), and putative structure of the autoinhibited DFG-In substate adopted in the absence of phosphorylation, identified in molecular dynamics simulations (bottom). The Cα atom of the residue on the activation loop labeled with the spin probe is shown as a sphere in both panels. (C) Schematics of the four main activation states of AurA highlighting the complementary structural and dynamic changes triggered by Tpx2 binding and phosphorylation (pT288), and the dynamically quenched state that results when these factors act together, e.g. in PP6-mutated melanoma cells.