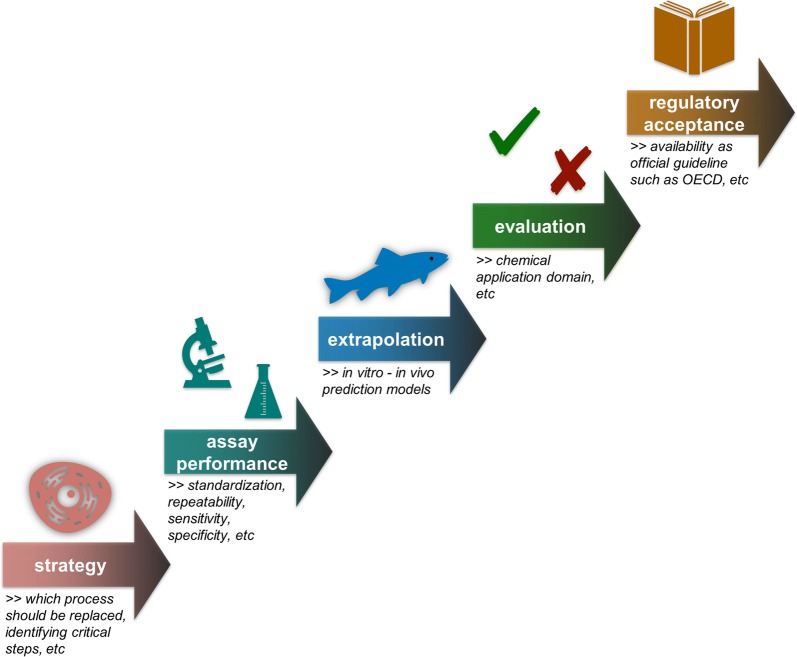
Fig. 2.
Crucial steps for developing and implementation of in vitro assays as an alternative for in vivo tests: First, steps and processes need to be defined in order to find out which target process could be replaced by in vitro assays. Next, assay performances including aspects such as test standardization have to be optimized, and it needs the development of appropriate in vitro–in vivo extrapolation models. Finally, the chemical application domain of the in vitro assays has to be identified. The last step, which is important for regulatory acceptance, is the implementation of an official Test Guideline, like the OECD Test Guidelines