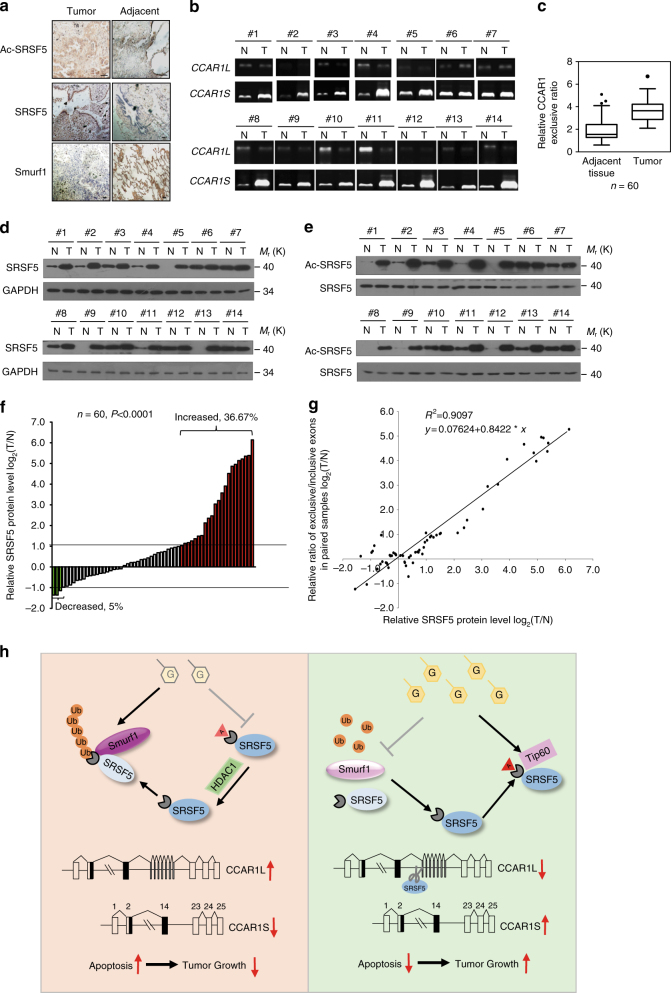
Fig. 10.
SRSF5 status correlates with CCAR1 splicing and tumorigenesis. a Representative images from immune-histochemical staining of Ac-SRSF5, SRSF5, and Smurf1 in three serial sections of the same tumor and matched adjacent tissue. Scale bar, 50 μm. b Total RNAs from 60 paired human NSCLC (T) and normal tissues (N) were examined by RT-PCR. Representative results for detection of CCAR1 exons 15–22 splicing patterns are shown. c Quantification of data from b for exons 15–22 exclusion ratio. The median box and whiskers plot was then calculated for the paired normal and tumor sets using Wilcoxon matched pairs test (*P < 0.05, one-way ANOVA test). d Lung cancer clinical cases with an increase in SRSF5 protein level. Human lung carcinoma samples paired with carcinoma tissue (shown as T) and adjacent normal tissue (shown as N) were lysed. The total SRSF5 protein levels were analyzed by immunoblotting analysis. e Lung cancer clinical cases with increased SRSF5 acetylation level at K125 in SRSF5-upregulated NSCLC. Human lung carcinoma samples paired with carcinoma tissue (shown as T) and adjacent normal tissue (shown as N) were lysed. The acetylated protein levels were compared against SRSF5 in immunoblotting analysis. f Relative expression of SRSF5 protein level in paired human clinical lung cancer samples and normal tissues. Immunoblotting analysis was performed on 60 paired human clinical lung cancer samples. Expression levels of SRSF5 were normalized to that of GAPDH. Data were calculated from triplicates. Bar value is the log ratio of SRSF5 expression levels between lung cancer samples (T) and matched normal tissues (N) from the same patient. Bar value ≤ −1 represents SRSF5 is decreased in tumors. Bar value > 1 represents that SRSF5 is increased in tumors. g Positive correlation between CCAR1 exclusive exons 15–22/inclusive exons 15–22 ratio and expression levels of SRSF5 was observed in human clinical lung samples. Relationships between these two variables were determined by Pearson’s correlation coefficients. h Model for ubiquitylation and acetylation of SRSF5 regulating alternative splicing of CCAR1 in signaling glucose sufficiency. Unprocessed original scans of blots are shown in Supplementary Fig. 9