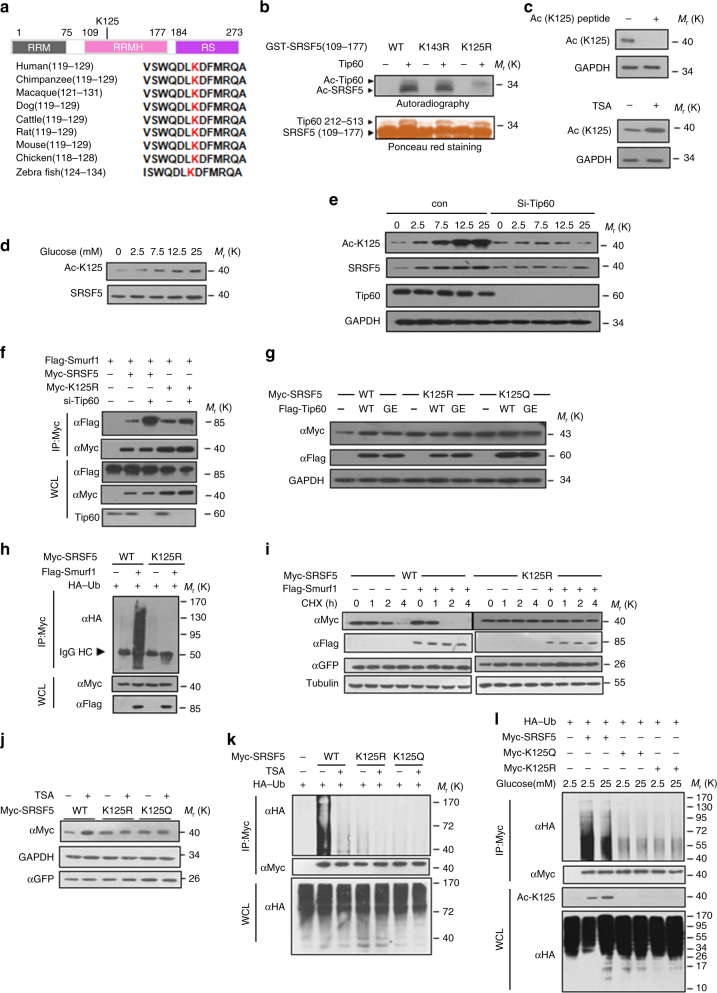
Fig. 7.
Acetylation of SRSF5 protects it from degradation. a Multiple sequences alignments of SRSF5 across species. b K125 is a prime-candidate site for Tip60-mediated acetylation as detected by autoradiography. c Confirmation of Ac-K125 antibody activity. d Glucose increases SRSF5 K125 acetylation level. The loading was normalized to SRSF5 protein levels so as to indicate the relative acetylation level. e Tip60 is required for glucose-regulated SRSF5 acetylation. Endogenous basic and acetylated level of SRSF5 in control and Tip60 knockdown cells in response to different glucose concentration were detected by immunoblotting. f Inhibition of Tip60 increases the interaction between Smurf1 and wild-type SRSF5, but not K125R. HEK293T cells treated with or without Tip60 were transfected with indicated plasmids. The interaction between SRSF5 and Smurf1 was determined by IP-western. g Myc-tagged SRSF5 WT, K125R, K125Q plasmids were co-expressed for 36 h in A549 cells with either wild-type (WT) HA–Tip60 or mutant (G380E) HA–Tip60. Immunoblotting analysis using anti-Myc antibody is presented. h Amino acid K125 of SRSF5 is required for the ubiquitylation mediated by Smurf1 in vivo. HEK293T cells transfected with indicated plasmids were subjected to ubiquitylation analysis, as revealed by immunoblotting. i Substitution of SRSF5 lysine 125 to arginine prolongs its half-life. HEK293T cells were transfected with plasmids as indicated. Cells were subjected to CHX treatment for indicated times and the lysates were analyzed. j TSA treatment increases the abundance of SRSF5 WT but not K125 mutants. Myc-tagged SRSF5 K125 WT or mutant plasmids were transfected into HEK293T cells with or without TSA treatment. Expression of SRSF5 were analyzed by immunoblotting. k TSA decreases the ubiquitylation of SRSF5 WT, but not K125 mutants. HEK293T cells were transfected with indicated plasmids with or without TSA treatment. Ubiquitylation of purified proteins was analyzed. l High glucose decreases the ubiquitylation of SRSF5 WT, but not K125 mutants. HEK293T cells transfected with indicated plasmids were maintained under 2.5 or 25 mM glucose concentrations. Ubiquitylation and acetylation of purified proteins were analyzed. Data are representative of three independent biological replicates. Unprocessed original scans of blots are shown in Supplementary Fig. 9