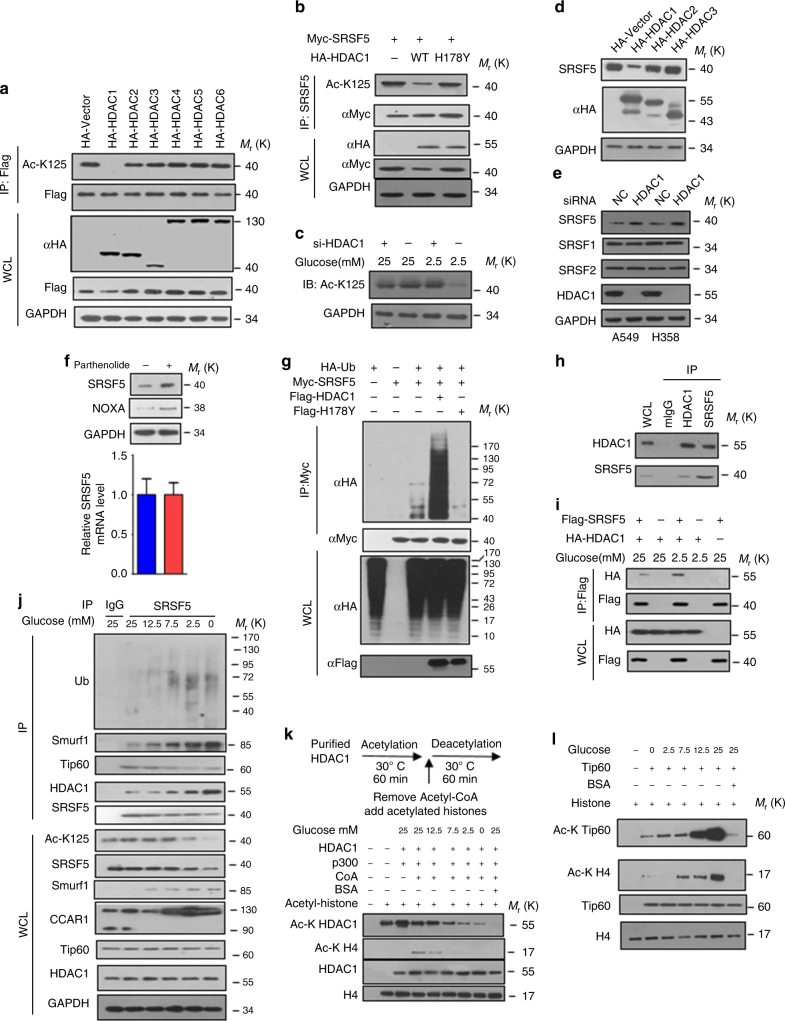
Fig. 8.
HDAC1 deacetylates SRSF5 upon low glucose. a HDAC1 overexpression specifically decreases SRSF5 acetylation. HEK293T cells were transfected with indicated plasmids and the acetylation levels of SRSF5 were determined by immunoblotting. b Catalytic activity of HDAC1 is required for the deacetylation of SRSF5. Myc-tagged SRSF5 was co-transfected with HA-tagged HDAC1 WT or catalytically inactive mutant H178Y into HEK293T cells. Acetylation was determined by immunoblotting. c HDAC1 is required for glucose-regulated SRSF5 acetylation. HEK293T cells transfected with or without siHDAC1 were cultured in medium containing 2.5 or 25 mM glucose. d Overexpression of HDAC1 specifically decreases SRSF5 protein level as revealed by immunoblot. e HDAC1 knockdown increases SRSF5 protein level in A549 and H358 cells. f A549 cells were treated with or without HDAC1 inhibitor Parthenolide. Endogenous SRSF5 protein levels were determined by immunoblot analysis and relative SRSF5 mRNA levels were quantified by qPCR. g HDAC1, but not its catalytic-inactive mutant, promotes SRSF5 ubiquitylation. HEK293T cells transfected with Myc-tagged SRSF5, Flag-tagged HDAC1 WT or H178Y vectors, HA-tagged ubiquitin were subjected to ubiquitylation analysis, as revealed by immunoblotting. h Endogenous interaction between SRSF5 and HDAC1 were revealed by co-immunoprecipitation assays. i High glucose decreases the interaction between HDAC1 and SRSF5. Flag-tagged SRSF5 were co-transfected with HA-tagged HDAC1 into HEK293T cells upon different glucose concentrations. Protein interactions were determined. j A549 cells were maintained at various glucose concentrations for 18 h and harvested for immunoprecipitation and immunoblotting analysis. k HDAC1 activity is inhibited by acetylation at high concentration. HDAC1 purified from HEK293T cells maintained at different concentrations of glucose was first acetylated with p300. Acetyl-CoA was then removed by dialysis, and the samples were incubated with acetylated core histones to examine the HDAC1 deacetylase activity. l Glucose protrusion promoted Tip60 autoacetylation. Tip60 purified from A549 cells maintained at indicated glucose concentration was incubated with H4, [3H]-acetyl CoA, or BSA (1 mg) as indicated, and the auto-acetylation was detected by immunoblotting. Data are representative of three independent biological replicates (f; mean and s.e.m., n = 3). Unprocessed original scans of blots are shown in Supplementary Fig. 9