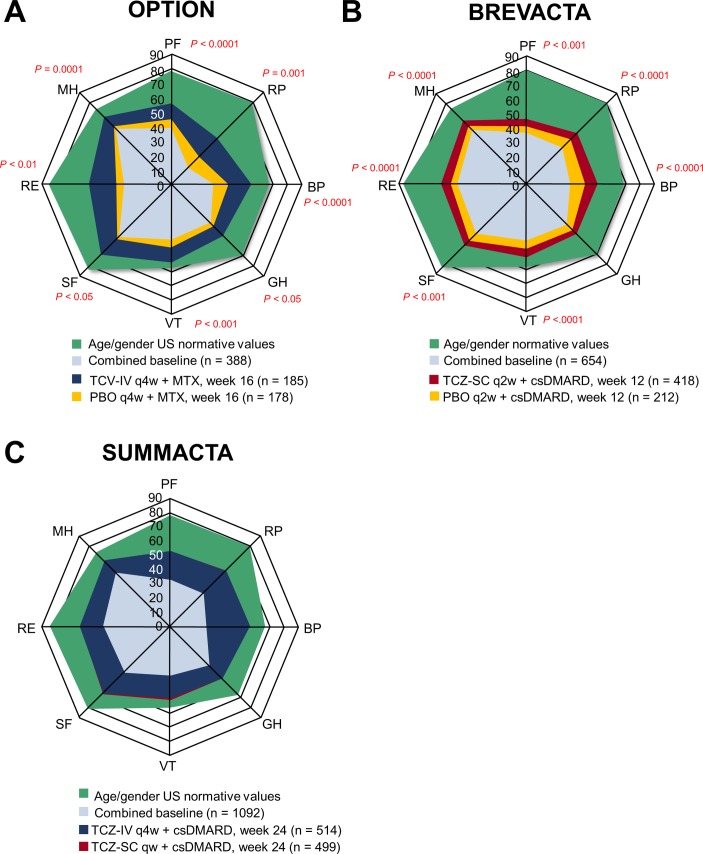
Figure 1.
SF-36 domain scores at baseline and at (A) 16 weeks in OPTION, (B) 12 weeks in BREVACTA and (C) 24 weeks in SUMMACTA compared with age and gender-matched normative values. Analyses were performed using the intention-to-treat population in OPTION (TCZ-intravenous, n=205; PBO, n=204), intention-to-treat population in BREVACTA (TCZ-subcutaneous, n=437; PBO, n=219) and per-protocol population in SUMMACTA (TCZ-subcutaneous, n=558; TCZ-intravenous, n=537). BP, bodily pain; csDMARD, conventional synthetic disease-modifying antirheumatic drug; GH, general health; IV, intravenous; MH, mental health; MTX, methotrexate; PBO, placebo; PF, physical functioning; qw, weekly; q2w, every 2 weeks; q4w, every 4 weeks; RE, role-emotional; RP, role-physical; SC, subcutaneous; SF, social functioning; SF-36, Short Form-36; TCZ, tocilizumab; VT, vitality.