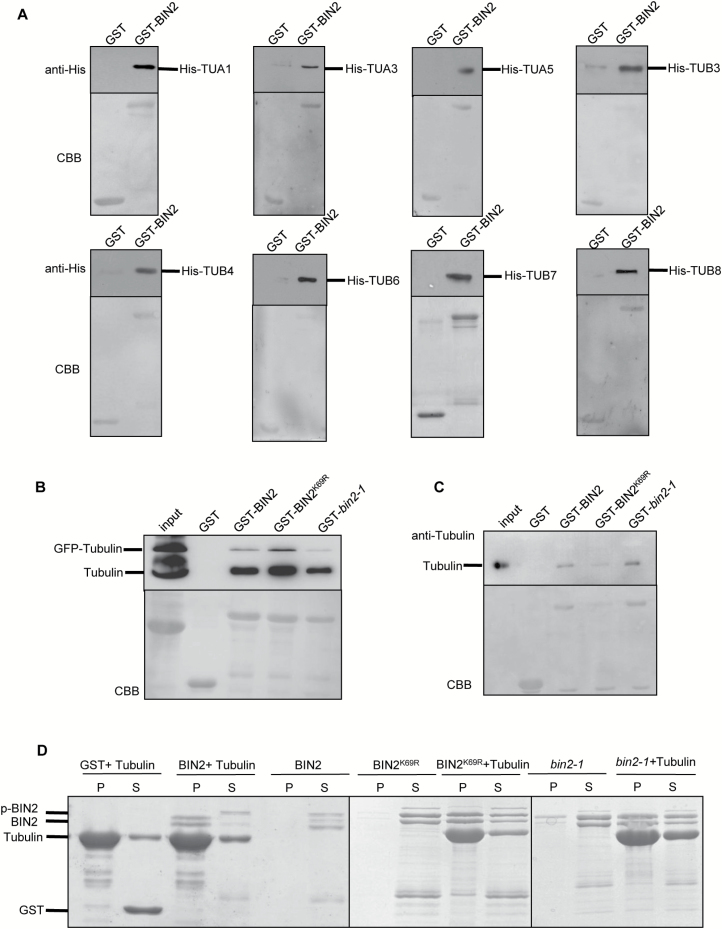
Fig. 5.
BIN2 interacts with tubulins in vitro and semi-in vivo. (A) BIN2 directly interacts with tubulins in vitro by pull-down assay. Tubulin–His was pulled down by GST–BIN2 immobilized on glutathione resin and analysed by western blotting using anti-His antibody. GST was used as a negative control. GST–BIN2 and tubulin–His were expressed and purified from E. coli. The data presented are a western blot (top) and a Coomassie-stained gel (bottom). (B) The interaction between BIN2 and tubulins was detected by a semi-in vivo pull-down assay. GST–BIN2 was expressed and purified from E. coli. Protein extracts from plants overexpressing tubulin–GFP were used for pull-down assays. Tubulin–GFP and tubulin were pulled down by GST–BIN2, GST–BIN2K69R and GST–bin2-1 immobilized on glutathione resin and analysed by western blotting using anti-α-tubulin antibody. GST was used as a negative control. The data presented are a western blot (top) and a Coomassie-stained gel (bottom). (C) The interaction between BIN2 and tubulins was tested by semi-in vivo pull-down assays. Tubulins were pulled down by GST–BIN2, GST–BIN2K69R, and GST–bin2-1 immobilized on glutathione resin and analysed by western blotting using anti-tubulin antibody. GST was used as a negative control. GST–BIN2 was expressed and purified from E. coli and tubulin was purified from porcine brain. The data presented are a western blot (top) and a Coomassie-stained gel (bottom). (D) The interaction between BIN2 and microtubules was detected by co-sedimentation assays. Recombinant GST–BIN2, GST–BIN2K69R, and GST–bin2-1 proteins were co-sedimented with 5 mM Taxol microtubules. Recombinant proteins appeared mainly in the supernatant (S) after centrifugation in the absence of microtubules and co-sedimented with microtubules into the pellets (P) in the presence of microtubules. GST was used as a negative control. (This figure is available in color at JXB online.)