Abstract
Background
Protein-energy wasting (PEW) in end-stage renal disease (ESRD) patients is associated with increased morbidity and mortality, but options for treatment are limited. Growth hormone (GH) increases insulin-like growth factor 1 (IGF-1), with improved nutritional parameters, but must be given subcutaneously and does not provide normal GH secretion patterns. MK-0677, an oral ghrelin receptor agonist (GRA), maintains normal GH secretion and increases lean body mass in normal subjects; it has not been studied in dialysis patients, an essential step in assessing efficacy and safety prior to clinical trials.
Methods
We performed a randomized crossover double-blind study in assessing the effect of MK-0677 versus placebo on IGF-1 levels, the primary outcome, in hemodialysis patients. In total, 26 subjects enrolled and 22 completed the 3-month crossover study.
Results
The geometric mean IGF-1 was 1.07-fold greater [95% confidence interval (CI) 0.89–1.27; P = 0.718] after placebo. In patients receiving MK-0677, the geometric mean IGF-1 were 1.76-fold greater (95% CI 1.48–2.10; P < 0.001) following MK-0677. When the data were adjusted for preintervention IGF-1 concentration, the ratio of geometric means (MK-0677 relative to placebo) for the pre- versus postintervention change in the IGF-1 was 1.65 (95% CI 1.33–2.04; P < 0.001). These data demonstrate a 65% greater increase (95% CI 33–104%) in IGF-1 in MK-0677-dosed subjects compared with placebo. There were no serious adverse effects attributable to MK-0677.
Conclusions
MK-0677 increased serum IGF-1 levels with minimal adverse effects in hemodialysis subjects. Studies are needed to evaluate whether long-term therapy with MK-0677 improves PEW, lean body mass, physical strength, quality of life and survival in CKD/ESRD patients.
Keywords: ESRD, ghrelin, growth hormone, IGF-1, protein-energy wasting
INTRODUCTION
Protein-energy wasting (PEW) is a common finding in end-stage renal disease (ESRD) patients and begins in chronic kidney disease (CKD). Half of patients with CKD have PEW, and this increases with ESRD [1–3]. Decreased albumin has been linked with future morbidity and mortality in ESRD patients [4–11]; prealbumin [12] and body mass index [13] have also been linked to adverse outcomes in ESRD. The etiologies of PEW in ESRD are diverse, including anorexia, uremic factors such as metabolic acidosis and increased levels of cytokines leading to increased catabolism as well as insulin resistance [14–19].
Current options for PEW intervention in ESRD have met with limited success. Options such as dietary counseling, appetite stimulants and dietary supplements often are insufficient to improve PEW. To date, the only intervention with data to suggest improvement is intradialytic parenteral nutrition [20]. Recent studies have assessed hormonal treatment of PEW in ESRD using either anabolic steroids or interventions on the growth hormone (GH)–insulin-like growth factor 1 (IGF-1) axis. These suggest that stimulation of the GH–IGF-1 axis can potentially improve PEW.
Ghrelin is an endogenous hormone that decreases acute and chronic inflammation, enhances the immune system, stimulates appetite and causes physiologic pulsatile release of GH. MK-0677 was developed by Merck as a high-affinity, long-acting, orally active GH secretagogue (GHS) [21]. MK-0677 was then used as a tool to clone its receptor, then known as the GH secretagogue receptor. This receptor was then used to identify its endogenous ligand, which resulted in the discovery of ghrelin [22]. This receptor has been renamed the ghrelin receptor and is the only known mechanism of action for both ghrelin and MK-0677. Studies have demonstrated that MK-0677, and also ghrelin, enhance the amplitude of endogenous pulses of GH secretion, resulting in increased levels of circulating IGF-1 [23]. MK-0677, an orally active ghrelin receptor agonist (GRA), is a GHS. It has been shown to increase pulsatile GH secretion in elderly patients [13, 24]. MK-0677 has not been previously assessed in an ESRD patient population. Endogenous GH secretion, unlike exogenous GH administration, is pulsatile and therefore time-of-draw dependent, while IGF-1 levels provide a constant indicator of GH secretion [23]. We hypothesized that this GRA would increase IGF-1 in ESRD patients on hemodialysis and could possibly improve their nutritional status. While MK-0677 has shown efficacy in normal subjects, it has not been shown to be efficacious or safe in dialysis patients. It is necessary to demonstrate efficacy and safety for a new agent in the vulnerable ESRD population before it can be assessed in long-term trials. We report here successful achievement of our primary goal of an increase in IGF-1 in response to GRA MK-0677, with minimal adverse effects.
MATERIALS AND METHODS
Design
This was a randomized crossover double-blind study. The protocol was reviewed by the General Clinic Research Center and the Institutional Review Board at the University of Virginia and was compliant with the Helsinki Accord. The trial is registered at ClinicalTrials.gov (identification number NCT 00395291; 1 November 2006). The trial design is provided in the Supplementary data. Enrolled patients gave written consent to participate in the study. Matching placebo and MK-0677 were provided by Merck Research Laboratories. Patients received 25 mg of MK-0677 daily or placebo. The study design was a 3-month crossover treatment. Subjects were randomized to receive MK-0677 either in month 1 or 3. After a 1-month washout period, patients were switched to the alternate regimen. The primary outcome was IGF-1 levels. Compliance was assessed by pill counts. Care providers and the study team were blinded to randomization. Subjects were seen, examined and queried for adverse events (AEs) at the beginning and end of each month. In addition to evaluation for AEs at each visit, subjects were queried by telephone interviews for AEs at the midpoint of each month and 2 weeks after the third period. A per-protocol analysis was performed (see details below).
Power analysis
This study was designed to have at least 0.80 statistical power (1−β) to detect a MK-0677 versus placebo IGF-1 geometric mean ratio of 1.48 with 22 individuals (11 individuals randomly assigned to receive placebo initially and 11 individuals randomly assigned to receive MK-0677 initially). Details related to the power analysis are provided in the Supplemental data.
Recruitment
Approximately 250 charts from three University of Virginia dialysis clinics were preliminarily reviewed for study criteria over a 14-month period. In total, 49 subjects met preliminary criteria and were consented. These subjects were screened by history and physical exam, initial chart review and laboratory testing to assess for the presence of preexisting conditions or underlying disease that would exclude the individual’s participation. Inclusion requirements were that subjects received long-term regular dialysis thrice weekly. Incident dialysis patients and those with dialysis vintage <3 months were not enrolled. Exclusion criteria are listed in Table 1. In total, 26 patients were enrolled.
Table 1.
Exclusion criteria
|
Randomization
The study biostatistician (J.P.) generated the randomization list prior to the onset of patient enrollment. In traditional two-period crossover design fashion, the sequential order of the treatments assigned to the first and second periods of the crossover design was randomly permuted. For 50% of the treatment assignment sequences (i.e. n = 13), placebo was assigned to the first crossover period and MK-0677 was assigned to the second crossover period, while for the remaining 50% of the treatment assignment sequences MK-0677 was assigned to the first crossover period and placebo assigned to the second crossover period.
In order to maintain treatment sequence balance with patient dropout, 10 replacement treatment sequence assignments were generated a priori. Patients who were designated as replacement patients for patients who withdrew from the study were assigned to the same treatment sequence as the patient they replaced.
Blinding
Placebo and MK-0677 provided by Merck Research Laboratories included a randomization identification number and masked treatment assignment (A or B) to which only Merck and the study biostatistician were unblinded. Patients as well as study personnel (i.e. principal investigator, co-investigators, clinical trial coordinator and laboratory technicians) were blind to the pill bottle contents.
Drug disbursement
Drug was released by a study pharmacist and dispensed by the study coordinator. Study compliance was assessed with pill counts (see Supplementary data).
Outcome measures
Laboratory values were collected at multiple points during the study (see Supplementary data for study procedures schedule details). As part of the prestudy screening, a comprehensive metabolic panel (CMP), hemoglobin A1c and complete blood count (CBC) were obtained in addition to other screening labs. At the monthly study visits and the post-study follow-up (visit 4), the following studies were obtained: interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α), IL-6, IL-10, high-sensitivity C-reactive protein (hsCRP), GH, IGF-1, insulin, leptin, ghrelin, esterase, adiponectin, CBC, comprehensive metabolic profile, albumin, prealbumin and metabolic chemistry (sodium, potassium, chloride bicarbonate, glucose, urea nitrogen). Thyroid function tests and liver function tests were performed in the Clinical Laboratory at the University of Virginia. Other studies were performed as described below. Blood and vital signs, including weight, were obtained immediately before the initiation of regularly scheduled treatments. Since subjects from all dialysis shifts participated, it was not possible to obtain fasting blood samples. Each cycle (placebo, washout and MK-0677) was coordinated with these blood draws (i.e. begun after the blood was obtained).
Hormone and cytokine assays
Determination of GH, IGF-1, insulin, hsCRP, IL-6, IL-10 and TNF-α are provided in the Supplementary data.
Assays for ghrelin and butyrylcholinesterase
We used two separate two-site sandwich assays, one specific for acyl ghrelin (AG) and one for des-acyl ghrelin (DG). These assays do not measure ghrelin fragments and have demonstrated superior specificity for AG and DG determination relative to single site assays [25, 26]. Total ghrelin was the sum of AG and DG. Butyrylcholinesterase (BuChE), which degrades ghrelin, was measured as described [25]. Details are provided in the Supplementary data.
Outcome data
The outcome data that were utilized in the statistical analyses represented the pre- to postintervention change in the outcome variable. With the exception of the data for body weight (kg), all of the outcome data were transformed to the natural logarithmic scale prior to computing the pre- to postintervention change. The logarithmic transformations were conducted as a consequence of exploratory analyses, which showed the logarithmic change to be more symmetrically distributed. The data for each outcome were analyzed via a conventional two-period crossover linear mixed model. The model specification details are provided in the Supplementary data.
Hypothesis testing
With regard to hypothesis testing, a linear contrast of means was constructed to formally test whether the mean pre- to post-intervention change in the outcome was equal to zero. Similarly, a linear contrast of means was constructed to test whether the mean pre- to postintervention change in the outcome was the same regardless of the intervention (MK-0677 or placebo). Each hypothesis was evaluated using a two-sided test and a P ≤ 0.05 decision rule.
CI construction
CI construction was based on the Student's t distribution. For those variables that were analyzed on the natural logarithmic scale, the lower and upper limits of the CI were exponentiated to obtain a 95% CI for the ratio of geometric means.
RESULTS
Details of the results of the laboratory studies and AEs are presented in the Supplementary data, as well as individual dialysis vintage, diagnoses and compliance. The baseline characteristics of the patients are provided in Table 2. Of 49 subjects screened, 26 were enrolled from June 2008 to January 2009. Two males and two females dropped out. Twenty-two subjects completed the study, for a dropout rate of 15.4%. There were 17 African American and 5 Caucasian subjects. There was a wide range of ages enrolled in the study. The size of the study population precluded meaningful subgroup analysis. Ninety-five percent of subjects were receiving an erythropoietic agent, antihypertensive and vitamin D, while 91% were on phosphate binders, 86% on dietary supplements and 77% were receiving various medications for gastrointestinal symptoms. Other medications for other symptoms were used less frequently. The primary and secondary diagnoses for ESRD included hypertension in all 22, focal segmental glomerular sclerosis in 3, diabetes in 3, membranoproliferative glomerulonephritis in 1 and chronic interstitial nephritis in 1.
Table 2.
Baseline characteristics of those 22 patients who completed the trial prior to first admission
| Baseline variables | Summary |
|---|---|
| Gender (male) | 16 (72.7) |
| Age (years) | 53.0 (47.7–71.5) |
| Race (African American) | 17 (77.3) |
| Body weight (kg) | 77.7 (66.7–87.7) |
| Vintage (years) | 4.5 (1.3–7.8) |
| IGF-1 (ng/mL) | 117.5 (75.0–185.5) |
| GH (ng/mL) | 1.5 (0.8–3.6) |
| Leptin (ng/mL) | 4.2 (0.9–17.9) |
| Acyl ghrelin (pg/mL) | 38.4 (12.1–118.2) |
| Des-acyl ghrelin (pg/mL) | 210.9 (65.5–298.4) |
| Total ghrelin (pg/mL) | 321.0 (109.7–348.4) |
| Adiponectin (ng/mL) | 18 310.7 (11 968.1–28 805.3) |
| Insulin (mIU) | 16.4 (10.5–23.1) |
| hsCRP (mg/dL) | 5.1 (2.1–9.0) |
| IL-1β (pg/mL) | 0.25 (0.13–0.50) |
| IL-6 [AuthorQuery id="AQ7" rid="7"]?> (pg/mL) | 5.1 (2.9–8.0) |
| IL-10 (pg/mL) | 1.8 (1.2–3.2) |
| TNF-α (pg/ml) | 3.9 (2.9–4.6) |
| Esterase (U/mL) | 40.3 (353–47.5) |
Categorical variables presented as n (%). Continuous variables presented as median (interquartile range) of the empirical distribution.
GH and IGF-1
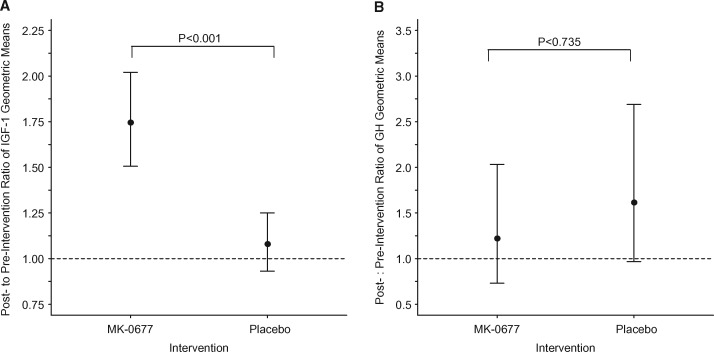
The geometric mean for IGF-1 concentration was 1.07-fold greater (95% CI 0.89–1.27; P = 0.718) following placebo dosing than before receiving placebo. In subjects receiving MK-0677, the geometric mean for IGF-1 concentration was 1.76-fold greater (95% CI 1.48–2.10; P < 0.001) following MK-0677 intervention (Figure 1A). When the data were adjusted for preintervention IGF-1 concentration, the ratio of geometric means (MK-0677 relative to placebo) for the pre- versus postintervention change in IGF-1 was 1.65 (95% CI 1.33–2.04; P < 0.001). These data demonstrate a 65% greater increase (95% CI 33–104%) in IGF-1 concentration in the MK-0677-dosed subjects at 30 days compared with placebo.
FIGURE 1.
Postintervention:preintervention ratio of IGF-1 and GH geometric means. Circles identify the geometric mean ratio and the vertical lines identify the 95% CIs for the geometric mean ratio. Hatch lines denote the reference line for a geometric mean ratio equal to 1. P-values correspond to the test of the null hypothesis that the ratio of geometric means is the same irrespective of the intervention.
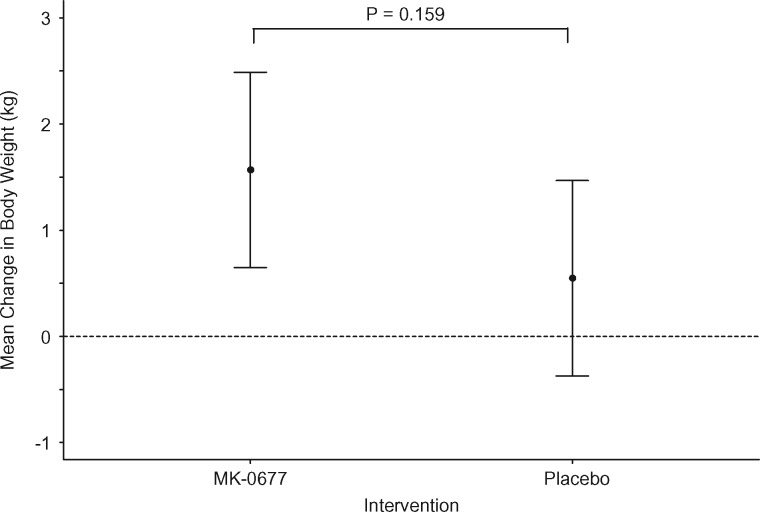
FIGURE 2.
Change in body weight from pre- to postintervention. Circles identify the mean change in body weight and the vertical lines identify the 95% CI for the mean change. Hatch lines denote the reference line for a mean change equal to 0. The P-value corresponds to the test of the null hypothesis that the mean change in body weight (kg) is the same irrespective of the intervention.
GH did not show a statistically significant change with either placebo or MK-0677. Neither the postintervention geometric mean for GH in the MK-0677 group nor the postintervention geometric mean for GH in the placebo group differed from the preintervention geometric mean (P = 0.437 and P = 0.066, respectively) . The ratios of post- to preintervention GH levels were 1.22 (95% CI 0.73, 2.03) and 1.61 (95% CI 0.97–2.69), respectively, for MK-0677 and placebo (P = 0.735; Figure 1B).
Blood glucose
Pre- to postintervention change in blood glucose differed between the MK-0677 and placebo interventions (P = 0.048). The geometric mean blood glucose increased by 31% (95% CI 11, 55; P = 0.003) while on MK-0677 compared with 0% (95% CI −15, 19; P = 0.977) while on placebo. However, the results may have been skewed by the inclusion of three type 2 diabetic patients (subjects 3, sulfonylurea; 5, diet control and 20, insulin). These individuals had increases of random blood glucose of 164, 246 and 301 mg/dL. A post hoc analysis excluding the three diabetic patients demonstrated that the pre- to postintervention change in blood glucose differed only marginally between the MK-0677 and placebo interventions (P = 0.068). For this subset of patients, geometric mean blood glucose increased by 12% (95% CI 2, 23; P = 0.020) while on MK-0677 compared with −3% (95% CI −12, 7; P = 0.564) while on placebo.
Other hormone and cytokine studies
The remainder of assessed hormones and cytokines did not demonstrate a statistically significant change after placebo or MK-0677 (Table 3).
Table 3.
Ratio of post- to preintervention geometric means for the secondary outcome variables
| MK-0677 |
Placebo |
||||
|---|---|---|---|---|---|
| Variable | Geometric mean ratio (95% CI) | P-value† | Geometric mean ratio (95% CI) | P-value† | P-value‡ |
| AG | 0.61 (0.40–0.92) | 0.020 | 0.95 (0.63–1.44) | 0.813 | 0.169 |
| DG | 1.07 (0.75–1.52) | 0.718 | 0.95 (0.67–1.35) | 0.733 | 0.782 |
| Total ghrelin | 0.92 (0.72–1.18) | 0.512 | 0.92 (0.72–1.18) | 0.485 | 0.900 |
| GH | 1.22 (0.73–2.03) | 0.437 | 1.63 (0.97–2.69) | 0.066 | 0.735 |
| Adiponectin | 1.05 (0.96–1.16) | 0.262 | 1.01 (0.92–1.11) | 0.876 | 0.545 |
| Insulin | 1.22 (0.94–1.59) | 0.131 | 0.93 (0.72–1.21) | 0.597 | 0.075 |
| hsCRP | 0.95 (0.59–1.53) | 0.841 | 0.99 (0.61–1.59) | 0.949 | 0.929 |
| IL-1β | 1.09 (0.81–1.46) | 0.557 | 0.95 (0.71–1.28) | 0.736 | 0.905 |
| IL-6 | 1.40 (1.00–1.95) | 0.049 | 1.08 (0.77–1.51) | 0.651 | 0.233 |
| IL-10 | 0.96 (0.73–1.25) | 0.734 | 1.20 (0.92–1.58) | 0.177 | 0.277 |
| TNF-α | 1.12 (0.82–1.53) | 0.450 | 1.04 (0.76–1.43) | 0.780 | 0.385 |
| Esterase | 0.95 (0.83–1.07) | 0.388 | 0.97 (0.86–1.10) | 0.668 | 0.875 |
P-value for the test of the null hypothesis that the ratio of the postintervention to preintervention geometric mean (post: pre) is equal to 1.
P-value for the test of the null hypothesis that the MK-0677 geometric mean ratio is equal to the placebo geometric mean ratio.
Body weight
There was an increase in weight with both MK-0677 and placebo. Weight increased by 1.6 kg with MK-0677 (95% CI 0.6, 2.5; P = 0.001) and 0.5 kg with placebo (95% CI −0.4–1.5; P = 0.237). However, there was not a statistically significant difference in weight change between MK-0677 and placebo (P = 0.159).
AEs
There were no serious AEs attributable to MK-0677. However, there were few subjects and the treatment time was short. See the Supplementary data for details of adverse events.
DISCUSSION
Resistance to the GH–IGF-1 axis has been documented in uremia and is consequent to multiple etiologies [27, 28, 29]. Various studies have examined the use of recombinant GH in ESRD patients and its effects on secondary markers of nutrition [30]. These have shown an increase in albumin and lean body mass and a decrease in protein catabolic rate. In addition, GH is used to increase growth in uremic children. A drawback to these studies includes the short time frame and small number of patients. In addition, GH replacement must be administered by subcutaneous injection, resulting in poor patient compliance, and it produces a single pharmacologic pulse in 24 h versus the normal physiologic pattern of 20–25 pulses in 24 h [31;32]. MK-0677 is not the same as GH. As a GRA, it induces secretion of GH and also preserves the physiological pattern of GH secretion, unlike exogenously administered GH [23].
Ghrelin is a peptide that affects appetite and has anti-inflammatory properties. Ghrelin exists in two forms, acylated and des-acyl ghrelin. AG stimulates appetite and antagonizes leptin, which has a negative effect on appetite [33, 34]. DG has been reported to delay gastric emptying and causes a decrease in food intake [33, 35]. Ghrelin levels, specifically DG, are elevated in ESRD patients [36]. Ghrelin has been linked with anorexia of ESRD, which has been suggested to be due to the negative effects of DG [33, 34, 37], which can be removed by dialysis [38, 39].
The GRA MK-0677 has previously been shown to increase IGF-1 level in healthy and elderly patients with intact renal function [23, 40]. It binds to the ghrelin receptor GH secretagogue receptor and mimics the effect of AG. We have now documented the same effect in subjects with ESRD receiving traditional hemodialysis thrice weekly. Given this finding, it is possible that other primary and secondary outcomes assessed in studies on MK-0677 could be applied to the hemodialysis patient population as well.
Previous work on GH has suggested that supplementation of GH can have numerous effects on markers of PEW, bone disease, and lipid metabolism. Markers that have been specifically assessed include an increase in albumin, body mass and transferrin. GH has also been shown to reduce protein catabolic rate. Although our study was not designed to show these effects, demonstration that MK-0667 can increase IGF-1 levels would suggest that in an expanded study it may provide these same effects. This is also suggested by the increase in blood glucose with MK-0677 in the present study, consistent with a GH effect. Larger studies will be needed to assess any detrimental effects of MK-0677 on glucose.
Ghrelin also has effects not related to GH activities. The GRA activity of MK-0677 might thus provide similar results for ESRD patients. In several small studies, administration of ghrelin to ESRD patients with PEW increased food intake [24, 41]. Ghrelin also increases fat stores compared with GH, which is lipolytic. This is important to consider given data that increased fat stores improve outcomes in ESRD [42]. Previous studies of MK-0677 in elderly patients documented an increase in limb fat [23]. Ghrelin also has potent anti-inflammatory properties, promotes lymphocyte development in bone marrow and thymus and decreases age-related thymic involution [43]. These properties are especially relevant in the CKD/ESRD population. Finally, low IGF-1 levels are associated with increased mortality in dialysis patients independent of biomarkers of PEW [44]. MK-0677 increases IGF-1 levels. While GH treatment did not improve survival in ESRD subjects in previous studies, these may have been underpowered [45].
The relationship between AG and DG in ESRD patients is important to consider. No effect was seen on either hormone in this study with placebo or MK-0677. The actions of MK-0677 as a GRA suggest its actions in ESRD patients are consistent with AG. The effects of increasing appetite and antagonizing leptin could be beneficial in ESRD patients with PEW. No effect was seen on leptin concentrations in this study, but an antagonistic effect on leptin could benefit malnourished and anorexic ESRD patients.
Our study has limitations. Its duration was short and the sample was small, limiting assessment of safety in this population. The population was heavily weighted to African American men. There could have been a carryover effect when the MK-0677 group crossed over to placebo. However, the plasma half-life of MK-0677 is only 6–13 h (investigators brochure). We also saw no evidence of a biologic carryover effect (Supplementary data). Finally, we previously reported that IGF-1 levels returned to pretreatment levels within 1 month after having received MK-0677 for a year [23].
This was a ‘proof-of-concept’ study to assess the effects of MK-0677 on the IGF-1 axis and examine short-term safety in ESRD patients. It showed that an oral GRA can increase serum IGF-1 levels in ESRD patients on hemodialysis thrice weekly. GH is known to be diabetogenic. We observed in this study, as expected and previously observed in other studies, that diabetic patients may need additional treatment to control their blood glucose while on MK-0677. Only the known diabetic patients had significant worsening of their random blood glucoses. The other subjects had a modest increase in blood glucose. No effect of MK-0677 on acyl, des-acyl and total ghrelin levels was observed. However, the samples were only drawn once pre- and once posttreatment and were not fasting, which is not ideal.
CONCLUSIONS
This study demonstrates a positive effect on IGF-1 by the GRA MK-0677. Stimulation of ghrelin receptors effected by MK-0677 has the potential for significant benefit for CKD/ESRD patients with PEW, where our treatment options are limited. Since MK-0677 is an oral agent, compliance is likely to be greater compared with subcutaneous injections required for GH or GH releasing factors such as AKL-0707 [46]. No toxicity was observed in our study, in contrast to the oral anabolic steroid oxymetholone, despite its positive anabolic effects [47]. Further studies will need to be conducted to determine the clinical effects of MK-0677 in CKD/ESRD.
SUPPLEMENTARY DATA
?>Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
Thanks to the Nephrology Clinical Research Center, the University of Virginia General Clinical Research Center, and to Lisa Johnson for trial coordination.
We appreciate the administrative assistance of Anita Jacobson for preparation of the manuscript.
FUNDING
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R21DK077372 and Merck.
This work was supported in part by a National Institutes of Health grant to the University of Virginia General Clinical Research Center (M01RR000847).
CONFLICT OF INTEREST STATEMENT
At the time of the study none of the authors had any competing financial interests. Since the performance of the study, one author (M.O.T.) founded Ammonett Pharma, which is now developing MK-0677.
REFERENCES
- 1. Ikizler TA. Nutrition, inflammation and chronic kidney disease. Curr Opin Nephrol Hypertens 2008; 17: 162–167 [DOI] [PubMed] [Google Scholar]
- 2. Muscaritoli M, Molfino A, Bollea MR. et al. Malnutrition and wasting in renal disease. Curr Opin Clin Nutr Metab Care 2009; 12: 378–383 [DOI] [PubMed] [Google Scholar]
- 3. Fouque D, Pelletier S, Mafra D. et al. Nutrition and chronic kidney disease. Kidney Int 2011; 80: 348–357 [DOI] [PubMed] [Google Scholar]
- 4. Fleischmann E, Teal N, Dudley J. et al. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int 1999; 55: 1560–1567 [DOI] [PubMed] [Google Scholar]
- 5. Goldwasser P, Mittman N, Antignani A. et al. Predictors of mortality in hemodialysis patients [abstract]. J Am Soc Nephrol 1993; 3: 1613–1622 [DOI] [PubMed] [Google Scholar]
- 6. Barrett BJ, Parfrey PS, Morgan J. et al. Prediction of early death in end-stage renal disease patients starting dialysis. Am J Kidney Dis 1997; 29: 214–222 [DOI] [PubMed] [Google Scholar]
- 7. Marcen R, Teruel JL, de la Cal MA. et al. The impact of malnutrition in morbidity and mortality in stable haemodialysis patients. Spanish Cooperative Study of Nutrition in Hemodialysis. Nephrol Dial Transplant 1997; 12: 2324–2331, [DOI] [PubMed] [Google Scholar]
- 8. Soucie JM, McClellan WM.. Early death in dialysis patients: risk factors and impact on incidence and mortality rates. J Am Soc Nephrol 1996; 7: 2169–2175 [DOI] [PubMed] [Google Scholar]
- 9. Iseki K, Uehara H, Nishime K. et al. Impact of the initial levels of laboratory variables on survival in chronic dialysis patients. Am J Kidney Dis 1996; 28: 541–548 [DOI] [PubMed] [Google Scholar]
- 10. Avram MM, Mittman N, Bonomini L. et al. Markers for survival in dialysis: a seven-year prospective study. Am J Kidney Dis 1995; 26: 209–219 [DOI] [PubMed] [Google Scholar]
- 11. Owen WF Jr, Lew NL, Liu Y. et al. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med 1993; 329: 1001–1006 [DOI] [PubMed] [Google Scholar]
- 12. Chertow GM, Ackert K, Lew NL. et al. Prealbumin is as important as albumin in the nutritional assessment of hemodialysis patients. Kidney Int 2000; 58: 2512–2517 [DOI] [PubMed] [Google Scholar]
- 13. Huang CX, Tighiouart H, Beddhu S. et al. Both low muscle mass and low fat are associated with higher all-cause mortality in hemodialysis patients. Kidney Int 2010; 77: 624–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lowrie EG, Lew NL.. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis 1990; 15: 458–482 [DOI] [PubMed] [Google Scholar]
- 15. Guarnieri G, Antonione R, Biolo G.. Mechanisms of malnutrition in uremia. J Ren Nutr 2003; 13: 153–157 [DOI] [PubMed] [Google Scholar]
- 16. Kalantar-Zadeh K, Stenvinkel P, Pillon L. et al. Inflammation and nutrition in renal insufficiency. Adv Ren Replace Ther 2003; 10: 155–169 [DOI] [PubMed] [Google Scholar]
- 17. Chandra RK. Impact of nutritional status and nutrient supplements on immune responses and incidence of infection in older individuals. Ageing Res Rev 2004; 3: 91–104 [DOI] [PubMed] [Google Scholar]
- 18. Marcos A, Nova E, Montero A.. Changes in the immune system are conditioned by nutrition. Eur J Clin Nutr 2003; 57: S66–S69. [DOI] [PubMed] [Google Scholar]
- 19. Mak RH, Cheung W, Cone RD. et al. Orexigenic and anorexigenic mechanisms in the control of nutrition in chronic kidney disease. Pediatr Nephrol 2005; 20: 427–431 [DOI] [PubMed] [Google Scholar]
- 20. Smith RG. Development of growth hormone secretagogues. Endocr Rev 2005; 26: 346–360 [DOI] [PubMed] [Google Scholar]
- 21. Smith RG, Van der Ploeg LH, Howard AD. et al. Peptidomimetic regulation of growth hormone secretion. Endocr Rev 1997; 18: 621–645 [DOI] [PubMed] [Google Scholar]
- 22. Kojima M, Hosoda H, Date Y. et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999; 402: 656–660 [DOI] [PubMed] [Google Scholar]
- 23. Nass R, Pezzoli SS, Oliveri MC. et al. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults. Ann Intern Med 2008; 149: 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wynne K, Giannitsopoulou K, Small CJ. et al. Subcutaneous ghrelin enhances acute food intake in malnourished patients who receive maintenance peritoneal dialysis: a randomized, placebo-controlled trial. J Am Soc Nephrol 2005; 16: 2111–2118 [DOI] [PubMed] [Google Scholar]
- 25. Liu J, Prudom CE, Nass R. et al. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab 2008; 93: 1980–1987, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prudom C, Liu J, Patrie J. et al. Comparison of competitive radioimmunoassays and two-site sandwich assays for the measurement and intrepretation of plasma ghrelin levels. J Clin Endocrinol Metab 2010; 95: 2351–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johannsson G, Ahlmen J.. End-stage renal disease: endocrine aspects of treatment. Growth Horm IGF Res 2003; 13(Suppl A): S94–S101 [DOI] [PubMed] [Google Scholar]
- 28. Mahesh S, Kaskel F.. Growth hormone axis in chronic kidney disease. Pediatr Nephrol 2008; 23: 41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clemmons DR. Role of IGF binding proteins in regulating metabolism. Trends Endocrinol Metab 2016; 27: 375–391 [DOI] [PubMed] [Google Scholar]
- 30. Feldt-Rasmussen B, Lange M, Sulowicz W. et al. Adult Patients in Chronic Dialysis (APCD) Study Group: growth hormone treatment during hemodialysis in a randomized trial improves nutrition, quality of life, and cardiovascular risk. J Am Soc Nephrol 2007; 18: 2161–2171 [DOI] [PubMed] [Google Scholar]
- 31. Kopple JD, Cheung AK, Christiansen JS. et al. OPPORTUNITY™: a large-scale randomized clinical trial of growth hormone in hemodialysis patients. Nephrol Dial Transplant 2011; 26: 4095–4103 [DOI] [PubMed] [Google Scholar]
- 32. Mehls O, Schaefer F.. Missed OPPORTUNITY: growth hormone therapy in adults with CKD. Nephrol Dial Transplant 2011; 26: 3835–3837 [DOI] [PubMed] [Google Scholar]
- 33. Chen CY, Inui A, Asakawa A. et al. Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology 2005; 129: 8–25 [DOI] [PubMed] [Google Scholar]
- 34. Muscaritoli M, Molfino A, Chiappini MG. et al. Anorexia in hemodialysis patients: the possible role of des-acyl ghrelin. Am J Nephrol 2007; 27: 360–365 [DOI] [PubMed] [Google Scholar]
- 35. Jarkovska Z, Hodkova M, Sazamova M. et al. Plasma levels of active and total ghrelin in renal failure: a relationship with GH/IGF-1 axis. Growth Horm IGF Res 2005;15: 369–376 [DOI] [PubMed] [Google Scholar]
- 36. Chang CC, Hung CH, Yen CS. et al. The relationship of plasma ghrelin level to energy regulation, feeding and left ventricular function in non-diabetic haemodialysis patients. Nephrol Dial Transplant 2005; 20: 2172–2177 [DOI] [PubMed] [Google Scholar]
- 37. Asakawa A, Inui A, Fujimiya M. et al. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut 2005; 54: 18–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoshimoto A, Mori K, Sugawara A. et al. Plasma ghrelin and desacyl ghrelin concentrations in renal failure. J Am Soc Nephrol 2002; 13: 2748–2752 [DOI] [PubMed] [Google Scholar]
- 39. Gupta R, Kuppusamy T, Patrie JT. et al. Association of plasma des-acyl ghrelin levels with CKD. Clin J Am Soc Nephrol 2013; 8: 1098–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bach MA, Rockwood K, Zetterberg C. et al. The effects of MK-0677, an oral growth hormone secretagogue, in patients with hip fracture. J Am Geriatr Soc 2004; 52: 516–523 [DOI] [PubMed] [Google Scholar]
- 41. Ashby DR, Ford HE, Wynn KJ. et al. Sustained appetite improvement in malnourished dialysis patients by daily ghrelin treatment. Kidney Int 2009; 76: 199–206 [DOI] [PubMed] [Google Scholar]
- 42. Cheung W, Yu PX, Little BM. et al. Role of leptin and melanocortin signaling in uremia-associated cachexia. J Clin Invest 2005; 115: 1659–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baatar D, Patel K, Taub D.. The effects of ghrelin on inflammation and the immune system. Mol Cell Endocrinol 2011; 340: 44–58 [DOI] [PubMed] [Google Scholar]
- 44. Nilsson E, Carrero JJ, Heimburger O. et al. A cohort study of insulin-like growth factor 1 and mortality in haemodialysis patients. Clin Kidney J 2016; 9: 148–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kopple JD, Cheung AK, Christiansen JS. et al. OPPORTUNITY: a randomized clinical trial of growth hormone on outcome in hemodialysis patients. Clin J Am Soc Nephrol 2008; 3: 1741–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Niemczyk S, Sikorska H, Wiecek A. et al. A super-agonist of growth hormone-releasing hormone causes rapid improvement of nutritional status in patients with chronic kidney disease. Kidney Int 2009; 77: 450–458. [DOI] [PubMed] [Google Scholar]
- 47. Supasyndh O, Satirapoj B, Aramwit P. et al. Effect of oral anabolic steroid on muscle strength and muscle growth in hemodialysis patients. Clin J Am Soc Nephrol 2013; 8: 271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.