Abstract
Immuno-proteomic screening has identified several tumor-associated auto-antibodies (AAb) that may have diagnostic capacity for invasive epithelial ovarian cancer, with AAbs to P53 proteins and cancer-testis antigens (CTAGs) as prominent examples. However, the early detection potential of these AAbs has been insufficiently explored in prospective studies.
We performed ELISA measurements of AAbs to CTAG1A, CTAG2, P53, and NUDT11 proteins, for 194 patients with ovarian cancer and 705 matched controls from the European EPIC cohort, using serum samples collected up to 36 months prior to diagnosis under usual care. CA125 was measured using electrochemo-luminiscence. Diagnostic discrimination statistics were calculated by strata of lead-time between blood collection and diagnosis. With lead times ≤6 months, ovarian cancer detection sensitivity at 0.98 specificity (SE98) varied from 0.19 [95% CI 0.08–0.40] for CTAG1A, CTAG2 and NUDT1 to 0.23 [0.10–0.44] for P53 (0.33 [0.11–0.68] for high-grade serous tumors). However, at longer lead-times the ability of these AAb markers to distinguish future ovarian cancer cases from controls declined rapidly; at lead times >1 year, SE98 estimates were close to zero (all invasive cases, range: 0.01–0.11). Compared to CA125 alone, combined logistic regression scores of AAbs and CA125 did not improve detection sensitivity at equal level of specificity. The added value of these selected AAbs as markers for ovarian cancer beyond CA125 for early detection is therefore limited.
Keywords: early detection, antibodies, prospective validation
Introduction
Cancer antigen 125 [CA125] is the best available biomarker for epithelial ovarian cancer, and the only marker tested in prospective screening trials so far. In randomized trials, however, the combination of CA125 with trans-vaginal ultrasonography (TVUS) provided either no reduction in ovarian cancer mortality (Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial [PLCO], USA)1, or only a suggestive mortality reduction using the Risk of Ovarian Cancer Algorithm (“ROCA”) algorithm, based on longitudinal changes in CA125 in serial measurements over time (United Kingdom Collaborative Trial on Ovarian Cancer Screening [UKCTOCS])2. CA125 has relatively low sensitivity for ovarian cancer early detection, particularly for early stage disease3 or in serum samples taken more than 6 months prior to symptomatic diagnosis4, 5, prompting searches for complementary biomarkers that can detect ovarian cancer in earlier clinical stages and at longer lead-times prior to usual symptomatic diagnosis.
A promising class of novel markers for early cancer detection is auto-antibodies [AAbs] against mutant, aberrantly post-processed or locally over-expressed proteins in tumors6–8. Through replication of antibody producing B-cells, AAbs could amplify a signal from antigens at very low concentrations, and at an early stage in tumorigenesis when the corresponding antigens may not themselves be detectable in the circulation.
To date, more than 80 AAbs have been investigated for ovarian cancer detection9. In our own work, we have successfully discovered first sets of AAbs with high tumor specificity among ovarian cancer patients10–12. In multi-stage discovery studies, using programmable protein microarrays containing 5,177 and 10,247 candidate antigens we identified sets of three and eleven AAbs, respectively, that were significantly associated with invasive ovarian cancer. Among these, antibodies against p53, the cancer/testis antigen CTAG-2 (also known as ESO2), and NUDT11 stood out as AAb markers with highest diagnostic sensitivity (up to 27.3 and 36.4%, respectively for serous tumors) at ≥97% specificity. A further AAb frequently reported to be associated with ovarian cancer13,9 and other tumors types14, 15, is CTAG1A (also known as NY-ESO-01). However, with the exception of two recent studies on AAbs against MUC1 (Ca15.3)16 and p5317, the early detection potential of tumor associated AAbs for ovarian cancer has been insufficiently evaluated in prospective cohort studies based on pre-diagnostic serum samples, and it is still unclear whether elevated AAb levels can be used to reliably detect ovarian cancer ahead of usual diagnosis.
To further examine the capacity of AAbs to provide early detection signals for ovarian cancer, as a possible complement to CA125, we performed a prospective analysis on a selected panel of four AAbs – against P53, CTAG1A, CTAG2 and NUDT11 – within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, using serum samples collected up to 36 months before diagnosis of 194 ovarian cancer patients and 705 matched control participants.
Materials and Methods
Case-control study within the EPIC cohort
We conducted a case-control study nested within the EPIC cohort – a population-based, multicenter prospective cohort study in 10 European countries – a further extension of an earlier study on CA125 and other early detection markers for ovarian cancer4, 5. The present study includes pre-diagnostic serum samples from all incident cases (N=197) of epithelial invasive ovarian (ICD-O code: C569), fallopian tube (C570) or peritoneal cancers (C480, C481, C482, C488) according to the International Classification of Diseases for Oncology (ICD-O) with available data on tumor histology, and diagnosed within maximally 36 months after blood donation. All ovarian cancer cases had been ascertained prospectively through record linkage with cancer and pathology registries (all countries except France, Germany, Greece and Naples Italy), or through active follow-up and systematic verification of self-reports by detailed examination and coding of clinical records (France, Germany, Greece, and Naples, Italy). Information of tumor stage was available in part from pathology reports and in part from cancer registries, and for uniformity was coded either local disease (stage I), or high-stage disease (regionally spread or metastatic). Information on tumor characteristics (histologic subtype [serous, endometrioid, clear cell, mucinous, not otherwise specified (NOS), grade [well, moderately or poorly/undifferentiated] was additionally obtained from pathology reports. Well differentiated tumors were classified as low grade, whereas moderately and poorly/undifferentiated tumors were classified as high grade. Data on tumor histology were available for all 197 cases, whereas data on tumor grade and stage were available for 133 (68%) and 180 (91%) of the cases, respectively.
For each of the 197 case subjects up to four control participants (N=725) were randomly selected among appropriate risk sets consisting of all female cohort members with a blood sample, alive and free of cancer at the time of diagnosis of the index case. An incidence density sampling protocol was used, such that, in principle, control participants could include women who became a cancer case later in time and each control participant could be sampled more than once; however, none of the control participants have subsequently been identified as ovarian cancer cases. Case and control participants were matched on study recruitment center, age at blood donation (±6 months), time of the day of blood collection (±1 h), fasting status (<3 h, 3–6 h, >6 h), follow-up time, and menopausal status at blood collection, use of oral contraceptives or post-menopausal hormone replacements at the time of blood draw, and phase of menstrual cycle for premenopausal women.
Laboratory assays
Serum samples were analyzed in batches, sorted by study center and with samples from matched case-control sets together in the same batch. Measurements of CA125 were performed in the Genital Tract Biology Lab at Brigham Women’s Hospital, Boston, using a highly sensitive electrochemo-luminiscence (ECL) detection platform (Meso Scale Discovery, MSD), following methods described in detail previously5. Measurements of AAbs were performed at Virginia G. Piper Center for Personal Diagnostics, Biodesign Institute, Arizona State University, using Rapid Antigenic Protein In situ Display (RAPID) ELISA as previously described18. Antigen proteins were expressed as c-terminal GST fusion proteins using 1-Step Human Coupled in vitro Expression system (Thermo Scientific) and added to 96 well plates. Patient serum was diluted 1:100 in blocking buffer, and bound IgG antibody was detected using HRP conjugated goat anti-human IgG (Jackson ImmunoResearch Laboratories) and Supersignal ELISA Femto Chemiluminescent Substrate (Thermo Scientific). Relative light unit (RLU) ratios were calculated using the RLU of a specific antigen divided by the RLU of the control GST-protein. All assays were performed in duplicate and the average level was used. All samples were blinded to the investigators. Measurements of CA125 and AAbs were completed for a total of 194 incident cases of invasive ovarian cancer and 705 matched, cancer-free control participants. Missing values were due to insufficient sample volume for the AAb assays (6 samples, including 2 cases), and to missing data for previous measurements of CA125 (1 further case and 16 further controls).
Statistical analyses
Detection sensitivities were calculated at quantitative marker cut-off points corresponding to 95% (SE95) and 98% (SE98) specificity, respectively, determined on raw and adjusted biomarker values among all control participants (N=705).
The biomarker values were separately adjusted through linear regression models, fitted to the full control population, using country, age at blood donation, menopausal status and use of either oral contraceptives (OC) or menopausal hormone replacement therapy (HRT) at blood draw as predictors. The linear adjustment models were applied to all sample subjects and the markers’ residuals added to the markers’ overall mean values, before further analyses by unconditional logistic regression. As findings from adjusted and un-adjusted marker analyses were practically identical, only the results from unadjusted analyses are presented.
Logistic regression modelling was used for further analyses of receiver operating characteristic (ROC) curves and C-statistics, and to examine the discrimination capacity of multiple markers in combination. For multi-marker discrimination models, the statistical fit of nested models was compared using likelihood-ratio tests. In ROC analyses, the area under curve (AUC; also referred to as concordance [C-]statistic) was calculated as an overall measure for the markers’ capacity to discriminate future cancer cases from participants.
All analyses were performed by strata of lag-time (≤6, >6–12, >12–24, and >24–36 months), and were conducted in SAS, version 9.4 (SAS Institute, Cary, NC, USA).
Informed consent and data protection
All EPIC study participants had given their consent for future analyses of their blood samples for research purposes, and the present study was approved by the IARC Ethics Committee and the Institutional Review Boards of Brigham and Women’s Hospital and of the University of Heidelberg.
Results
For the 194 ovarian cancer cases and 705 matched control participants with complete biomarker measurements, baseline and tumor characteristics are presented in Table 1. Overall, the median age at cancer diagnosis was 59 years (range: 31–79 years). Of the 194 cancer cases, 187 (96%) had the ovary classified as primary tumor site, whereas in 4 (2%) the primary site reported was the fallopian tube and in 3 patients (1.5%) it was the peritoneum. More than half of the tumors (56%; n=108) were of serous histology. Of the 178 cases with stage data available, 32 were diagnosed with localized disease, whereas the remainder (N=146) were coded as having advanced (regionally spread and/or metastatic) disease. Of the patients with information on tumor grade, 14 (7%) were well-differentiated (“low-grade”) and 117 were moderately or poorly differentiated (“high-grade”). Cross-classifications of ovarian tumor histology by stage (spread) and tumor grade at diagnosis, and by lag time since blood donation, are shown in the on-line Supplementary Table S1.
Table 1.
Characteristics [median (min-max) or n (%)] of cases and controls at baseline [blood donation], and tumor characteristics of the ovarian cancer cases.
| Cases (N= 194) | Controls (N=705) | |
|---|---|---|
| Baseline Characteristics | ||
| Age at blood donation (range), years | 57.7 (30.3 – 76.8) | 57.8 (30.3 –77.6) |
| Premenopausal | 34 (18%) | 131 (19%) |
| Perimenopausal / undetermined | 25 (13%) | 84 (12%) |
| Postmenopausal | 135 (70%) | 490 (70%) |
| BMI, kg/m2 | 25.06 (17.27 – 44.18) | 24.94 (14.88 – 45.09) |
| Smoking: Never | 115 (59%) | 411 (58%) |
| Former | 34 (17%) | 144 (20%) |
| Current | 40 (21%) | 139 (20%) |
| Missing | 5 (3%) | 11 (2%) |
| Parity: 1 Child | 31 (16%) | 108 (15%) |
| 2 children | 59 (30%) | 242 (34%) |
| 3 or more children | 53 (27%) | 231 (33%) |
| Missing: | 11 (6%) | 44 (6%) |
| Hysterectomy (“yes”) | 11 (7%) | 62 (11%) |
| Characteristics of Cancer Cases | ||
| Age at diagnosis (range), years | 59.00 (31 – 79) | |
| Lag time since blood donation (range), months | 17.5 (0.5 – 36) | |
| Cancer Site | ||
| Ovary | 187 (96%) | |
| Fallopian Tube | 4 (2%) | |
| Peritoneum | 3 (2%) | |
| Histology | ||
| Serous | 108 (56%) | |
| Mucinous | 18 (9%) | |
| Endometrioid | 25 (13%) | |
| Clear Cell | 4 (2%) | |
| NOS | 33 (17%) | |
| Other | 6 (3%) | |
| Cancer grade | ||
| Well differentiated | 14 (7%) | |
| Moderately differentiated | 50 (26%) | |
| Poorly differentiated / undifferentiated | 67 (35%) | |
| Missing | 63 (32%) | |
| Disease spread | ||
| Localized | 32 (17%) | |
| Regionally spread and / or metastatic | 146 (75%) | |
| Missing | 16 (8%) | |
Adjusting for age and study center, partial (Spearman) correlation analyses revealed no significant associations between CA125 and any of the AAb markers among the controls; however, among the cases there were weak but significant associations of CA125 with AAbs against CTAG1A (r=0.17) and p53 (r=0.18). Cross-sectional analyses revealed no strong correlations (all estimated values <0.13) for any of the AAbs with age or menopausal status at blood draw, parity, age at last child birth, estimated lifetime number of ovulatory cycles, BMI, smoking, or serum levels of C-reactive protein (CRP) as a biomarker of inflammation status (results not shown).
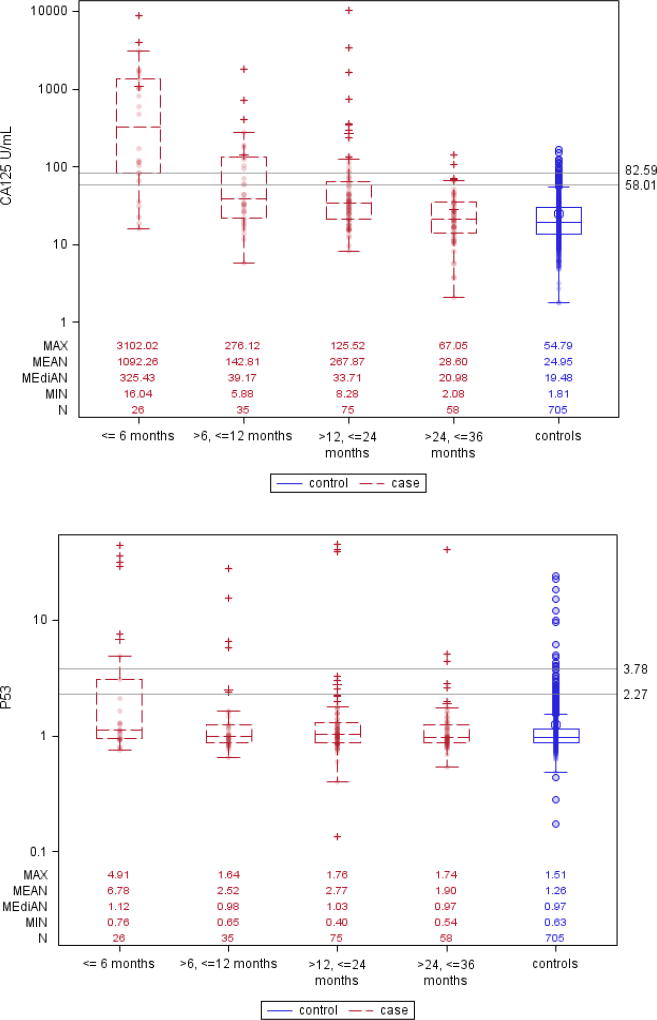
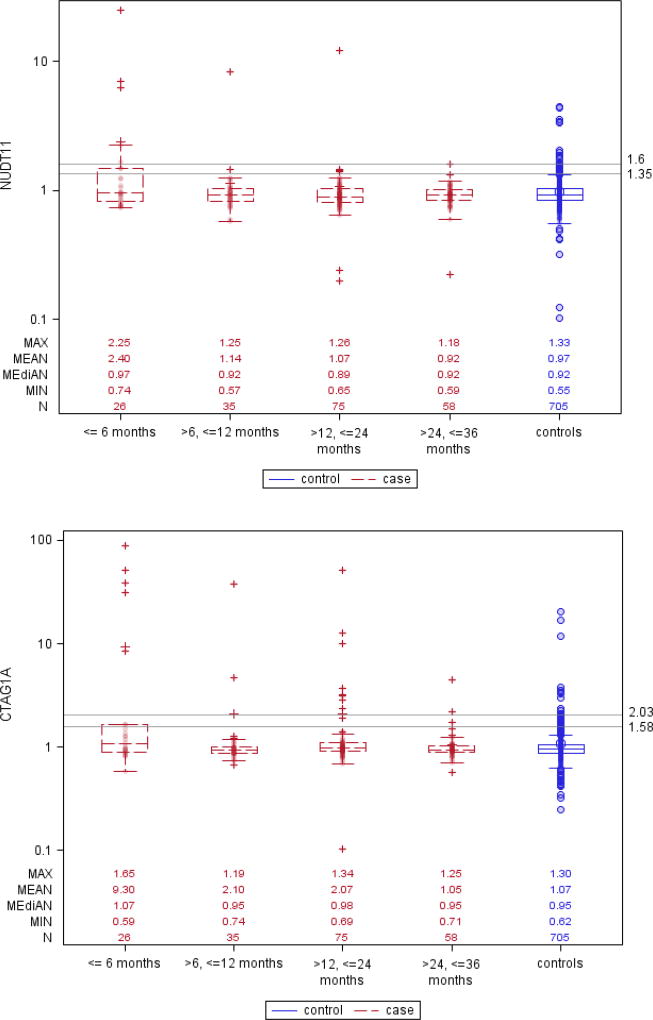
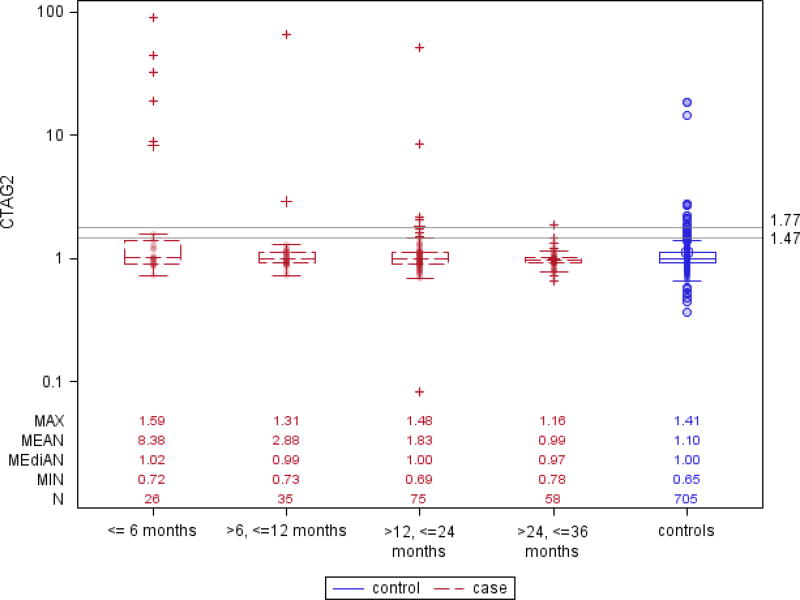
Box and whisker plots in (Figure 1) show that CA125 levels started diverging between future cases and control participants about 24 months prior to clinical diagnosis, and this difference grew larger as the lag-time diminished to 6 months or less, with a corresponding increase in the proportion of cases with marker levels above the 95% or 98% specificity cut-points. For each of the AAb markers the plots show similar trends of increasing proportion of ovarian cancer cases with elevated AAb titers as lag-times shortened, although absolute numbers of cases reaching threshold titers for 95% or 98% detection specificity were modest. Interestingly, the Box and whisker plots also showed elevated right-tail AAb titers in non-negligible proportions of cancer-free control participants.
Figure 1. Box and whisker plots showing serum levels of CA125, and antibody titers against CTAG1A, GTAG2, NUDT11 and P53, for ovarian cancer cases and matched controls, by intervals of time prior to diagnosis.
Reference lines are drawn at levels of the markers’ 95% and 98% specificity in all controls.
Using a quantitative marker cut-point corresponding to 98% specificity, CA125 showed sensitivity estimates (SE98) of 0.77, 0.34 and 0.20, respectively, for lag-times ≤6, >6–12, and >12–24 months, whereas for lag-times >24–36 months the sensitivity (SE98) was close to zero (0.03) (Table 2). For the AAb markers, estimates of SE98 ranged from 0.19 (CTAG1A, CTAG2, NUDT11) to 0.23 (P53) within the first 6 months after blood donation, from 0.03 (CTAG1A, NUDT11) to 0.11 (P53) for serum samples taken >6–12 months prior to diagnosis, and from 0.01 (NUDT11) to 0.11 (CTAG1A) for serum samples drawn >12–24 months prior to diagnosis. Using more lenient 95% specificity cut-points, the estimated sensitivities (SE95) were slightly higher.
Table 2.
Sensitivity at 95% (SE95) and 98% (SE98) specificity of ovarian cancer detection by time between blood donation and diagnosis
| All Ovarian Cancer (N=194) | High-Grade Serous Cancer (N= 75) | |||||
|---|---|---|---|---|---|---|
| Lead time (months) |
# Cases |
SE95 (95% CI) b | SE98 (95% CI) b | # Cases |
SE95 (95% CI) b | SE98 (95% CI) b |
| CA125 | ||||||
| ≤ 6 | 26 | 0.81 (0.60–0.92) | 0.77 (0.56–0.90) | 9 | 0.89 (0.49–0.98) | 0.89 (0.49–0.99) |
| >6–12 | 35 | 0.43 (0.27–0.61) | 0.34 (0.19–0.53) | 12 | 0.33 (0.13–0.63) | 0.33 (0.13–0.64) |
| ≤ 12 | 61 | 0.59 (0.45–0.72) | 0.52 (0.38–0.67) | 21 | 0.57 (0.35–0.77) | 0.57 (0.34–0.77) |
| >12–24 | 75 | 0.27 (0.17–0.39) | 0.20 (0.11–0.33) | 31 | 0.23 (0.11–0.41) | 0.13 (0.05–0.31) |
| ≤ 24 | 136 | 0.41 (0.31–0.52) | 0.35 (0.24–0.46) | 52 | 0.37 (0.24–0.52) | 0.31 (0.18–0.47) |
| >24–36 | 58 | 0.10 (0.04–0.22) | 0.03 (0.01–0.14) | 23 | 0.13 (0.04–0.34) | 0.04 (0.01–0.26) |
| ≤ 36 | 194 | 0.32 (0.24–0.41) | 0.25 (0.17–0.35) | 75 | 0.29 (0.19–0.42) | 0.23 (0.13–0.36) |
| P53 | ||||||
| ≤ 6 | 26 | 0.27 (0.13–0.48) | 0.23 (0.10–0.44) | 9 | 0.33 (0.11–0.67) | 0.33 (0.11–0.68) |
| >6–12 | 35 | 0.14 (0.06–0.31) | 0.11 (0.04–0.28) | 12 | 0.25 (0.08–0.56) | 0.17 (0.04–0.49) |
| ≤ 12 | 61 | 0.20 (0.11–0.33) | 0.16 (0.08–0.30) | 21 | 0.29 (0.13–0.52) | 0.24 (0.10–0.48) |
| >12–24 | 75 | 0.08 (0.03–0.18) | 0.04 (0.01–0.13) | 31 | 0.03 (0.00–0.20) | 0.03 (0.00–0.21) |
| ≤ 24 | 136 | 0.13 (0.08–0.21) | 0.10 (0.05–0.18) | 52 | 0.13 (0.06–0.27) | 0.12 (0.05–0.25) |
| >24–36 | 58 | 0.09 (0.03–0.20) | 0.05 (0.02–0.16) | 23 | 0.09 (0.02–0.30) | 0.09 (0.02–0.30) |
| ≤ 36 | 194 | 0.12 (0.07–0.19) | 0.08 (0.04–0.15) | 75 | 0.12 (0.06–0.23) | 0.11 (0.05–0.22) |
| CTAG1A | ||||||
| ≤ 6 | 26 | 0.27 (0.13–0.48) | 0.19 (0.08–0.40) | 9 | 0.44 (0.17–0.75) | 0.22 (0.05–0.59) |
| >6–12 | 35 | 0.06 (0.01–0.21) | 0.06 (0.01–0.21) | 12 | 0.00 (0.00–0.26) | 0.00 (0.00–0.26) |
| ≤ 12 | 61 | 0.15 (0.07–0.27) | 0.11 (0.05–0.24) | 21 | 0.19 (0.07–0.42) | 0.10 (0.02–0.32) |
| >12–24 | 75 | 0.12 (0.06–0.23) | 0.11 (0.05–0.22) | 31 | 0.16 (0.07–0.34) | 0.16 (0.06–0.35) |
| ≤ 24 | 136 | 0.13 (0.08–0.21) | 0.11 (0.06–0.20) | 52 | 0.17 (0.09–0.31) | 0.13 (0.06–0.27) |
| >24–36 | 58 | 0.05 (0.02–0.15) | 0.03 (0.01–0.14) | 23 | 0.04 (0.01–0.26) | 0.04 (0.01–0.26) |
| ≤ 36 | 194 | 0.11 (0.07–0.17) | 0.09 (0.05–0.16) | 75 | 0.13 (0.07–0.24) | 0.11 (0.05–0.22) |
| CTAG2 | ||||||
| ≤ 6 | 26 | 0.23 (0.10–0.44) | 0.19 (0.08–0.40) | 9 | 0.22 (0.05–0.58) | 0.22 (0.05–0.59) |
| >6–12 | 35 | 0.03 (0.00–0.18) | 0.03 (0.00–0.19) | 12 | 0.00 (0.00–0.26) | 0.00 (0.00–0.26) |
| ≤ 12 | 61 | 0.11 (0.05–0.23) | 0.10 (0.04–0.22) | 21 | 0.10 (0.02–0.32) | 0.10 (0.02–0.32) |
| >12–24 | 75 | 0.11 (0.05–0.21) | 0.05 (0.02–0.15) | 31 | 0.16 (0.07–0.34) | 0.10 (0.03–0.27) |
| ≤ 24 | 136 | 0.11 (0.06–0.19) | 0.07 (0.04–0.15) | 52 | 0.13 (0.06–0.27) | 0.10 (0.04–0.23) |
| >24–36 | 58 | 0.03 (0.01–0.13) | 0.02 (0.00–0.12) | 23 | 0.04 (0.01–0.26) | 0.04 (0.01–0.26) |
| ≤ 36 | 194 | 0.09 (0.05–0.15) | 0.06 (0.03–0.11) | 75 | 0.11 (0.05–0.21) | 0.08 (0.03–0.18) |
| NUDT11 | ||||||
| ≤ 6 | 26 | 0.27 (0.13–0.48) | 0.19 (0.08–0.40) | 9 | 0.33 (0.11–0.67) | 0.22 (0.05–0.59) |
| >6–12 | 35 | 0.06 (0.01–0.21) | 0.03 (0.00–0.19) | 12 | 0.00 (0.00–0.26) | 0.00 (0.00–0.26) |
| ≤ 12 | 61 | 0.15 (0.07–0.27) | 0.10 (0.04–0.22) | 21 | 0.14 (0.05–0.37) | 0.10 (0.02–0.32) |
| >12–24 | 75 | 0.07 (0.03–0.16) | 0.01 (0.00–0.09) | 31 | 0.10 (0.03–0.27) | 0.03 (0.00–0.21) |
| ≤ 24 | 136 | 0.10 (0.06–0.18) | 0.05 (0.02–0.12) | 52 | 0.12 (0.05–0.24) | 0.06 (0.02–0.18) |
| >24–36 | 58 | 0.02 (0.00–0.12) | 0.02 (0.00–0.12) | 23 | 0.00 (0.00–0.15) | 0.00 (0.00–0.15) |
| ≤ 36 | 194 | 0.08 (0.04–0.13) | 0.04 (0.02–0.09) | 75 | 0.08 (0.03–0.18) | 0.04 (0.01–0.13) |
Analyses of crude data, unadjusted for matching factors (see Methods section)
95% and 98% specificity cut-points were, respectively, 58.01 and 82.59 for CA125, 2.27 and 3.78 for P53-AAb, 1.47 and 1.77 for CTAG2-AAb, 1.58 and 2.03 for CTAG1A-AAb, 1.35 and 1.60 for NUDT11-AAb
When analyses were restricted to high-grade serous tumors, estimates for SE98 or SE95 were slightly higher for the AAb against P53 (e.g. SE98 = 0.33 and 0.17 for ≤6 and >6–12 months, respectively), but not for the other AAbs, whereas for all AAbs (including those to P53) early detection sensitivities (SE98 or SE95) remained practically zero for longer time lags (Table 2).
Among the control participants, a total of 61 women (8.6%) developed cancer over an extended follow-up of up to 20 years after blood donation, including one case of breast cancer within ≤36 months and one case of melanoma within ≤60 months. Excluding these control participants did not materially change estimates for 95% and 98% specificity cut-points, nor did it change estimates of SE98 or SE95 for early ovarian cancer detection.
Considering blood measurements ≤24 months before clinical diagnosis (the time frame within which marker discrimination could be most clearly observed), and using 98% specificity cut-points for each of the five markers, 47 out of 137 future cases of ovarian cancer (34%) showed positive test findings for CA125. Of the 89 CA125-negative cases, 8 (9%) would have been additionally detected through any one of the four AAbs. All 8 cases had blood samples predating clinical diagnosis by >6–24 months – a lead-time window in which the diagnostic sensitivity of CA125 was lower, and in which a larger proportion of tumors may have been still in earlier stages (Table 3). However, analyses among the control subjects showed that a combined diagnostic algorithm based on positive tests for either CA125 or any of the four AAbs would have also increased the false-positive detection rate [FPR] to 8.4%, and setting the quantitative specificity cut-point for CA125 to the same level yielded an equivalent increase in detection sensitivity for CA125 alone. Focusing on CTAG1A-AAb only, the one AAb marker that detected the largest proportion (6 of the 8) CA125-negative cases, the overall FPR for joint detection by either CA125 or AAb was lower (4.3%); still, the reduced panel of CA125 and CTAG1A-AAb did not outperform CA125 with a cut-point set at an equivalent FPR (e.g., sensitivity at FPR of 4.3% for lead time >12–24 months, CA125 or CTAG1A positive: 20%; CA125 alone: 19%) (Table 3). Similar results were observed for other marker combinations (Table 3) or with marker cut-points corresponding to either higher (99%) or lower (95%) levels of specificity.
Table 3.
Comparative sensitivity and specificity of early ovarian cancer detection using CA125 only, or a combined panel of CA125 with auto-antibodies (each marker individually dichotomized using its 98% specificity cut-point)a
| Lag Time (months) |
Cases | Controls testing marker positive (FPR %) |
Sensitivity for CA125 alone, at equivalent FPR cutpoint |
||||||
|---|---|---|---|---|---|---|---|---|---|
| # Cases |
CA125-positive; Sensitivity (%; cumulative %) a |
CA125 positive | CA125 negative | Total testing positive CA125 or AAb; Sensitivity (%; cumulative %) |
|||||
| AAb pos. b | AAb neg. | AAb pos. b | AAb neg. | ||||||
| CA125 + all four AAbs | |||||||||
| ≤6 | 26 | 20 (77%) | 20 | 0 | 0 | 6 | 20 (77%) | 59 / 705 (8.4%) | 21 (81%) |
| >6–12 | 35 | 12 (34%; 52%) | 12 | 0 | 2 | 21 | 14 (40%; 56%) | 15 (43%; 59%) | |
| >12–24 | 75 | 15 (20%; 35%) | 15 | 0 | 6 | 54 | 21 (28%; 40%) | 23 (31%; 43.4%) | |
| >24–36 | 58 | 5 (9%; 27%) | 2 | 0 | 5 | 51 | 7 (12%; 32%) | 7 (12%; 34%) | |
| CA125 + at least two AAbs positive (out of four) | |||||||||
| ≤6 | 26 | 20 (77%) | 5 | 15 | 0 | 6 | 20 (77%) | 25 / 705 (3.5%) | 20 (77%) |
| >6–12 | 35 | 12 (34%; 52%) | 0 | 12 | 1 | 22 | 13 (37%; 54%) | 13 (37%; 54%) | |
| >12–24 | 75 | 15 (20%; 35%) | 2 | 13 | 2 | 58 | 17 (23%; 37%) | 18 (24%; 38%) | |
| >24–36 | 58 | 5 (9%; 27%) | 0 | 2 | 2 | 54 | 4 (7%; 28%) | 3 (5%; 28%) | |
| CA125 + CTAG1A | |||||||||
| ≤6 | 26 | 20 (77%) | 5 | 15 | 0 | 6 | 20 (77%) | 30 / 705 (4.3%) | 21 (81%) |
| >6–12 | 35 | 12 (34%; 52%) | 1 | 11 | 1 | 22 | 13 (37%; 54%) | 13 (37%; 56%) | |
| >12–24 | 75 | 15 (20%; 35%) | 3 | 12 | 5 | 55 | 20 (27%; 39%) | 19 (25%; 39%) | |
| >24–36 | 58 | 5 (9%; 27%) | 0 | 2 | 2 | 54 | 4 (7%; 29%) | 6 (10%; 30%) | |
| CA125 + CTAG2 | |||||||||
| ≤6 | 26 | 20 (77%) | 5 | 15 | 0 | 6 | 20 (77%) | 29 /705 (4.1%) | 21 (81%) |
| >6–12 | 35 | 12 (34%; 52%) | 0 | 12 | 1 | 22 | 13 (37%; 54%) | 13 (37%; 56%) | |
| >12–24 | 75 | 15 (20%; 35%) | 2 | 13 | 2 | 58 | 17 (23%; 37%) | 18 (24%; 38%) | |
| >24–36 | 58 | 5 (9%; 27%) | 0 | 2 | 1 | 55 | 3 (5%; 27%) | 6 (10%; 30%) | |
| CA125 + NUDT11 | |||||||||
| ≤6 | 26 | 20 (77%) | 5 | 15 | 0 | 6 | 20 (77%) | 29 / 705 (4.1%) | 21 (81%) |
| >6–12 | 35 | 12 (34%; 52%) | 0 | 12 | 1 | 22 | 13 (37%; 54%) | 13 (37%; 56%) | |
| >12–24 | 75 | 15 (20%; 35%) | 1 | 14 | 0 | 60 | 15 (20%; 35%) | 18 (24%; 38%) | |
| >24–36 | 58 | 5 (9%; 27%) | 0 | 2 | 1 | 55 | 3 (5%; 26%) | 6 (10%; 30%) | |
| CA125 + P53 | |||||||||
| ≤6 | 26 | 20 (77%) | 6 | 14 | 0 | 6 | 20 (77%) | 29 / 705 (4.1%) | 21 (81%) |
| >6–12 | 35 | 12 (34%; 52%) | 3 | 9 | 1 | 22 | 13 (37%; 54%) | 13 (37%; 56%) | |
| >12–24 | 75 | 15 (20%; 35%) | 2 | 13 | 1 | 59 | 16 (21%; 36%) | 18 (24%; 38%) | |
| >24–36 | 58 | 5 (9%; 27%) | 0 | 2 | 3 | 53 | 5 (9%; 28%) | 6 (10%; 30%) | |
sensitivity % within specific lag-time window, and cumulatively up to specific window included;
positive test for any of the four AAbs at 98% specificity
Still focusing on data for the first 24 months of prospective follow-up, when modelling all markers on a continuous (log2-transformed) scale by logistic regression the overall model fit improved significantly (p=0.003) when the four AAbs were added to a model including CA125, but with only very modest increases in AUC (from 0.78 for CA125 alone, to 0.80 for the full model) (Table 4A). A backward elimination data analysis strategy, eliminating markers not contributing significantly to the model at a significance level of p≤0.10, resulted in a model containing only CA125, CTAG1A and NUDT11 that retained most of the improvement in model fit and in the AUC. Entering the AAbs as variables dichotomized around their 98% specificity cut-points led to a similar model selection of CA125 plus CTAG1A only, with similarly modest increases in AUC (Table 4B). In none of the above models, however, was there any improvement in detection sensitivity at overall 95% or 98% specificity for the corresponding relative risk (logistic regression) scores, as compared to models based on CA125 alone.
Table 4.
Comparison of models for discrimination of future ovarian cancer cases from cancer-free control participants a
| Cumulative lag- time (months) |
# Case- Control Sets |
Model 1: CA125 | Model 2: CA125 + all 4 AAbs | Model 3: CA125 + Selected AAbs e | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | p b | SE98 c | AUC (95% CI) | p d | SE98 c | AUC (95% CI) | p f | SE98 c | ||
| (A) All markers as continuous, log2-transformed variables | ||||||||||
| <0.0001 | 0.003 | Selected markers: CA125, CTAG1A, NUDT11 | ||||||||
| ≤ 6 | 26 | 0.94 (0.90–0.99) | 0.77 | 0.94 (0.90–0.99) | 0.73 | 0.95 (0.90–0.99) | 0.0008 | 0.73 | ||
| ≤ 12 | 61 | 0.84 (0.79–0.90) | 0.54 | 0.84 (0.79–0.90) | 0.51 | 0.84 (0.79–0.90) | 0.52 | |||
| ≤ 24 | 136 | 0.78 (0.74–0.83) | 0.35 | 0.80 (0.76–0.84) | 0.35 | 0.79 (0.75–0.84) | 0.35 | |||
| ≤ 36 | 194 | 0.71 (0.67–0.75) | 0.27 | 0.73 (0.69–0.77) | 0.27 | 0.72 (0.68–0.76) | 0.27 | |||
| (B) CA125 as continuous log2-transformed variable; all AAbs dichotomized around their 98-specificity cut-points | ||||||||||
| <0.0001 | 0.014 | Selected markers: CA125 + CTAG1A | ||||||||
| ≤ 6 | 26 | 0.94 (0.90–0.99) | 0.77 | 0.94 (0.88–0.99) | 0.73 | 0.93 (0.88–0.98) | 0.0009 | 0.73 | ||
| ≤ 12 | 61 | 0.84 (0.79–0.90) | 0.54 | 0.84 (0.79–0.90) | 0.52 | 0.84 (0.78–0.89) | 0.52 | |||
| ≤ 24 | 136 | 0.78 (0.74–0.83) | 0.35 | 0.79 (0.75–0.83) | 0.38 | 0.79 (0.75–0.83) | 0.38 | |||
| ≤ 36 | 194 | 0.71 (0.67–0.75) | 0.27 | 0.72 (0.68–0.76) | 0.28 | 0.72 (0.68–0.76) | 0.29 | |||
Unconditional logistic regression, with adjustment for matching variables (age at blood donation, study center, menopausal status, use of oral contraceptives or HRT at time of blood donation), fitted to data of the first 24 months of follow-up
Log-likelihood test, comparing with empty model
Diagnostic sensitivity at 98% specificity for the marker-based logistic regression risk score (CA125 alone, or CA125 plus AAbs)
Log-likelihood test, comparing with model 1
After backwards elimination of AAb variables to contributing significantly to model fit (using P≤0.10 as criterion for keeping
Log-likelihood test, comparing with model 1
Discussion
In this prospective study, a panel of four selected tumor-associated autoantibodies showed selectivity, but limited sensitivity, for early detection of ovarian cancer, prior to diagnosis under usual care. In serum samples predating symptomatic diagnosis by less than 6 months, each individual AAb marker showed a diagnostic sensitivity of around 0.20 at 0.98 specificity (SE98), similar to levels of diagnostic sensitivity observed in cross-sectional comparisons between clinically diagnosed ovarian cancer patients and cancer-free controls9. However, the ability of these AAb markers to distinguish cases from controls declined rapidly with time between blood draw and diagnosis, and SE98 estimates were close to zero in serum samples collected at greater than 1-year lead times. These observations suggest that high AAb titers to these selected cancer-associated antigens may represent increasing tumor burden, possibly related to increasing inflammation and immune cell infiltration, and that serial measurements may be needed to improve diagnostic performance. Combined logistic regression scores of the AAbs and CA125 showed no meaningful improvement in diagnostic discrimination (AUCs, SE98) compared to CA125 alone, despite a statistically significant improvement in overall model fit.
The AAbs included in the present study were selected on the basis of their diagnostic performance in previous studies by both our own10–12, 14, 18, and other research groups13,19. Elevated serum P53 AAbs are observed in relation to several selected cancer types, including lung, breast and gastro-intestinal tumors6–8, and elevated AAb titers to P53 have also been observed in more than ten studies comparing ovarian cancer patients to cancer-free control subjects (reviewed in9). Generally, the studies on ovarian cancer reported higher prevalence of elevated P53 AAbs among patients with high-grade serous tumors, as compared to other tumor subtypes, as was also observed in the current study. The higher sensitivity and specificity of P53 AAb for high-grade serous tumors is likely related to the uniform occurrence of P53 mutations, with dysregulated P53 protein levels, in high-grade serous tumors. Like the P53 AAbs, elevated titers of AAbs to the cancer-testis antigens CTAG1A (NY-ESO-1) and CTAG2 (ESO2) have been observed in relation to a wide variety of cancer types6–8,14, 15, including ovarian cancer20,9,13, and are likely related to the generally less differentiated nature of cancer cells, with aberrant expression of proteins that normally are expressed only in embryonic tissue types. AAbs to NUDT11 were first discovered as ovarian autoantigens through our own immuno-proteomic screening of ovarian cancer patients and controls18.
In clinical studies comparing cancer patients (ovary and other organ sites) with cancer-free controls, strongly skewed distributions of AAbs with elevated right-tail values for cancer patients have suggested high cancer-diagnostic specificity of high antibody titers. However, for our selected panel of AAbs we also observed a non-negligible prevalence of elevated “right-tail” titers among control participants. Exclusion of controls with a cancer diagnosis during extended follow-up did not alter this pattern. Thus, our observations suggest that AAbs against P53, CTAG (“cancer-testis antigens”) or other antigens considered to be tumor associated may have lower cancer specificity than is generally assumed.
One other prospective evaluation of AAbs as early detection markers for ovarian cancer was reported recently for P53-AAbs17. This study by Yang et al. was based on analyses within the multimodal screening arm of UKCTOCS – a population-based, randomized trial of ovarian cancer screening among post-menopausal women in the United Kingdom -- and included 220 ovarian cancer cases with 1,053 serial serum samples collected up to 5 years prior to ovarian cancer diagnosis, and 619 age-matched ovarian cancer-free controls with sera collected annually (n=3,069 samples). The majority of the ovarian cancer cases (74.5%) were screen-detected using CA125 and the ROCA algorithm followed by TVUS; the remainder (25.5%) were screen negative cases. Applying a P53-AAb cut-point corresponding to 2.7% specificity, Yang et al. reported a positive P53 antibody signal in 20.7% of the screen-positive cases and 16.1% of the screen-negative cases. Further, among screen-positive cases, P53 was elevated an average of 9.2 months prior to detection by ROCA, or 8.1 months prior to elevated CA125 (>35 U/mL) alone. Likewise, a P53-AAb signal was also observed among 9 of the 56 screen-negative cases (16.1%). However, the authors did not report the overall false-positive rate associated with a diagnostic algorithm based on the combinations of P53-AAb with either ROCA or single-time elevation of CA125, nor did they report whether a similar improvement in OC detection could have been achieved on the basis of CA125 measurements only at an equivalent relaxation of specificity (i.e., using lower-specificity marker cut-points for either ROCA or single-time CA125). In our data, generated by a different ELISA assay method for P53-AAb, while we also observed positive AAb signals (notably against CTAG1A) in a proportion of future ovarian cancer testing negative by CA125, further analyses showed that diagnostic algorithms based on combinations of CA125 with AAbs did not actually outperform CA125 alone at equivalent false-positive detection rates.
As a further analysis within the UKCTOCS, Yang et al performed multivariate logistic regression and ROC curve analyses to examine combined detection capacity of CA125 (single-time measurement at [98.1% specificity] cut-point of 35 U/mL) and P53-AAb. As in our study, they observed a statistically significant improvement in model fit and a modest increase in overall AUC for the combined, two-marker model as compared to a model based on CA125 only. However, as in our data, there was no improvement in sensitivity at 98% specificity. Furthermore, ovarian cancer diagnoses in the UKCTOCS multi-modal screening arm were largely driven by ROCA analyses of longitudinal changes in CA125. This introduces a methodologic complication for analyses of a single measure of CA125 alone, given the ROCA algorithm has higher sensitivity than a one-time measurement of CA125, and may have effectively handicapped the performance of a single measure of CA125, with possible overestimation of the complementary detection potential for P53-AAb.
Detecting cancer sufficiently in advance of usual symptomatic diagnosis is generally expected to improve chances for successful surgical intervention and long-term survival. While our prospective analyses allowed an estimation of lead times for marker-based ovarian cancer detection, an intrinsic limitation of our and other prospective bio-banking studies is that no information is available about the patients’ tumor stages at the time they provided their blood sample. It thus remains speculative whether those patients whose tumors might have been detectable earlier would have had a survival benefit if actually diagnosed at that time point, or whether generally those tumors found to be marker-detectable were already more advanced-stage. Notwithstanding these limitations, our benchmarking of early detection performance of the AAbs relative to CA125, in terms of diagnostic sensitivity within selected windows of lead time, indicates that our selected AAbs may have only limited value as complementary serum markers for ovarian cancer screening. Also, as clinical studies have shown that CA125 has limited sensitivity for small and early-case ovarian tumors, clearly the inferior performance of the AAbs compared to CA125 especially for lead times above 6 months suggest that these AAbs would fail to provide a sufficiently early signal for ovarian tumors to allow a survival benefit.
In conclusion, our selected AAbs did not appear to provide a meaningful improvement over CA125 alone in in early detection of ovarian cancer. Furthermore, diagnostic discrimination of the AAbs appears to wane quickly with longer lead times between blood collection and diagnosis, suggesting that AAbs against these cancer-related antigens may have limited utility for detecting early lesions. An unexpected finding was the non-negligible prevalence of high AAb titers among the cancer-free controls, which appears to put a possible limit to the specificity of these AAbs as cancer detection markers.
Supplementary Material
Novelty and Impact.
Autoantibodies against tumor-related antigens are considered promising markers for early detection. Using pre-diagnostic blood samples of ovarian cancer cases and controls from the EPIC cohort, we examined the prospective detection capacity of antibodies to P53, CTAG1A, CTAG2 and NUDT11. Our findings indicate that these auto-antibodies signal ovarian tumors with only limited sensitivity and shorter lead times as compared to CA125, and do not support their use as complementary biomarkers for early ovarian cancer detection.
Acknowledgments
Funding
This study was funded by National Institutes of Health, grants NCI Early Detection Research Network, U01 CA117374 (K.S. Anderson) and R01 CA 158119 (D. W. Cramer). The coordination of EPIC is financially supported by the European Commission (DG-SANCO) and the International Agency for Research on Cancer. The national cohorts are supported by Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center (DKFZ), Federal Ministry of Education and Research (BMBF), Deutsche Krebshilfe, Deutsches Krebsforschungszentrum and Federal Ministry of Education and Research (Germany); the Hellenic Health Foundation (Greece); Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); the RIVM (National Institute for Public Health and the Environment, Bilthoven, the Netherlands) for the contribution to the EPIC-Study (data collection and continuing contribution to follow-up and maintenance of biobank); ERC-2009-AdG 232997 and Nordforsk, Nordic Centre of Excellence programme on Food, Nutrition and Health (Norway); Health Research Fund (FIS), PI13/00061 to Granada; PI13/01162 to EPIC-Murcia), Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, ISCIII RETIC (RD06/0020) (Spain); Swedish Cancer Society, Swedish Research Council and County Councils of Skåne and Västerbotten (Sweden); Cancer Research UK (14136 to EPIC-Norfolk; C570/A16491 and C8221/A19170 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk, MR/M012190/1 to EPIC-Oxford) (United Kingdom). For information on how to submit an application for gaining access to EPIC data and/or biospecimens, please follow the instructions at http://epic.iarc.fr/access/index.php
Abbreviations
- CA125
Cancer antigen 125
- TVUS
trans-vaginal ultrasonography
- PLCO
Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial
- ROCA
Risk of Ovarian Cancer Algorithm
- UKCTOCS
United Kingdom Collaborative Trial on Ovarian Cancer Screening
- AAbs
auto-antibodies
- EPIC
European Prospective Investigation into Cancer and Nutrition
- ECL
electrochemo-luminiscence
- MSD
Meso Scale Discovery
- RAPID
Rapid Antigenic Protein In situ Display
- RLU
Relative light unit
- OC
oral contraceptives
- HRT
hormone replacement therapy
- ROC
receiver operating characteristic
- AUC
area under curve
- CRP
C-reactive protein
- FPR
false-positive detection rate
Footnotes
Conflicts of interest: Karen Anderson declares consultancies to PROVISTA Diagnostics.
References
- 1.Buys SS, Partridge E, Greene MH, Prorok PC, Reding D, Riley TL, Hartge P, Fagerstrom RM, Ragard LR, Chia D, Izmirlian G, Fouad M, et al. Ovarian cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial: findings from the initial screen of a randomized trial. Am J Obstet Gynecol. 2005;193:1630–9. doi: 10.1016/j.ajog.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, Amso NN, Apostolidou S, Benjamin E, Cruickshank D, Crump DN, Davies SK, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet (London, England) 2016;387:945–56. doi: 10.1016/S0140-6736(15)01224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen JG, White M, Cruz A, Farias-Eisner R. In 2014, can we do better than CA125 in the early detection of ovarian cancer? World J Biol Chem. 2014;5:286–300. doi: 10.4331/wjbc.v5.i3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cramer DW, Bast RC, Jr, Berg CD, Diamandis EP, Godwin AK, Hartge P, Lokshin AE, Lu KH, McIntosh MW, Mor G, Patriotis C, Pinsky PF, et al. Ovarian cancer biomarker performance in prostate, lung, colorectal, and ovarian cancer screening trial specimens. Cancer prevention research (Philadelphia, Pa) 2011;4:365–74. doi: 10.1158/1940-6207.CAPR-10-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terry KL, Schock H, Fortner RT, Husing A, Fichorova RN, Yamamoto HS, Vitonis AF, Johnson T, Overvad K, Tjonneland A, Boutron-Ruault MC, Mesrine S, et al. A Prospective Evaluation of Early Detection Biomarkers for Ovarian Cancer in the European EPIC Cohort. Clin Cancer Res. 2016;22:4664–75. doi: 10.1158/1078-0432.CCR-16-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaenker P, Ziman MR. Serologic autoantibodies as diagnostic cancer biomarkers--a review. Cancer Epidemiol Biomarkers Prev. 2013;22:2161–81. doi: 10.1158/1055-9965.EPI-13-0621. [DOI] [PubMed] [Google Scholar]
- 7.Desmetz C, Mange A, Maudelonde T, Solassol J. Autoantibody signatures: progress and perspectives for early cancer detection. Journal of cellular and molecular medicine. 2011;15:2013–24. doi: 10.1111/j.1582-4934.2011.01355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W, Peng B, Lu Y, Xu W, Qian W, Zhang JY. Autoantibodies to tumor-associated antigens as biomarkers in cancer immunodiagnosis. Autoimmunity reviews. 2011;10:331–5. doi: 10.1016/j.autrev.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortner RT, Damms-Machado A, Kaaks R. Systematic review: Tumor-associated antigen autoantibodies and ovarian cancer early detection. Gynecol Oncol. 2017 doi: 10.1016/j.ygyno.2017.07.138. [DOI] [PubMed] [Google Scholar]
- 10.Katchman BA, Barderas R, Alam R, Chowell D, Field MS, Esserman LJ, Wallstrom G, LaBaer J, Cramer DW, Hollingsworth MA, Anderson KS. Proteomic mapping of p53 immunogenicity in pancreatic, ovarian, and breast cancers. Proteomics Clin Appl. 2016;10:720–31. doi: 10.1002/prca.201500096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson KS, Cramer DW, Sibani S, Wallstrom G, Wong J, Park J, Qiu J, Vitonis A, LaBaer J. Autoantibody signature for the serologic detection of ovarian cancer. J Proteome Res. 2015;14:578–86. doi: 10.1021/pr500908n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson KS, Wong J, Vitonis A, Crum CP, Sluss PM, Labaer J, Cramer D. p53 autoantibodies as potential detection and prognostic biomarkers in serous ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:859–68. doi: 10.1158/1055-9965.EPI-09-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szender JB, Papanicolau-Sengos A, Eng KH, Miliotto AJ, Lugade AA, Gnjatic S, Matsuzaki J, Morrison CD, Odunsi K. NY-ESO-1 expression predicts an aggressive phenotype of ovarian cancer. Gynecol Oncol. 2017;145:420–5. doi: 10.1016/j.ygyno.2017.03.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Figueroa JD, Wallstrom G, Barker K, Park JG, Demirkan G, Lissowska J, Anderson KS, Qiu J, LaBaer J. Plasma Autoantibodies Associated with Basal-like Breast Cancers. Cancer Epidemiol Biomarkers Prev. 2015;24:1332–40. doi: 10.1158/1055-9965.EPI-15-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang ZM, Ling ZG. Serum tumor-associated autoantibodies as diagnostic biomarkers for lung cancer: A systematic review and meta-analysis. 2017;12:e0182117. doi: 10.1371/journal.pone.0182117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burford B, Gentry-Maharaj A, Graham R, Allen D, Pedersen JW, Nudelman AS, Blixt O, Fourkala EO, Bueti D, Dawnay A, Ford J, Desai R, et al. Autoantibodies to MUC1 glycopeptides cannot be used as a screening assay for early detection of breast, ovarian, lung or pancreatic cancer. Br J Cancer. 2013;108:2045–55. doi: 10.1038/bjc.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang WL, Gentry-Maharaj A, Simmons A, Ryan A, Fourkala EO, Lu Z, Baggerly KA, Zhao Y, Lu KH, Bowtell D, Jacobs I, Skates SJ, et al. Elevation of TP53 Autoantibody Before CA125 in Preclinical Invasive Epithelial Ovarian Cancer. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-17-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katchman BA, Chowell D, Wallstrom G, Vitonis AF, LaBaer J, Cramer DW, Anderson KS. Autoantibody biomarkers for the detection of serous ovarian cancer. Gynecol Oncol. 2017;146:129–36. doi: 10.1016/j.ygyno.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirandola L, M JC, Cobos E, Bernardini G, Jenkins MR, Kast WM, Chiriva-Internati M. Cancer testis antigens: novel biomarkers and targetable proteins for ovarian cancer. International reviews of immunology. 2011;30:127–37. doi: 10.3109/08830185.2011.572504. [DOI] [PubMed] [Google Scholar]
- 20.Stockert E, Jager E, Chen YT, Scanlan MJ, Gout I, Karbach J, Arand M, Knuth A, Old LJ. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998;187:1349–54. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.