Highlights
-
•
Embryonal rhabdomyosarcoma of the uterine cervix and ovarian Sertoli-Leydig cell tumors are associated with DICER1 mutation
-
•
DICER1-associated tumors should prompt genetic counseling and testing
-
•
Somatic and germline genetic mutation profiles can be used to differentiate second primary from recurrent tumors
Keywords: DICER1 syndrome, Embryonal rhabdomyosarcoma, Sertoli-Leydig cell tumor
1. Introduction
While rhabdomyosarcoma is the most common pediatric soft tissue sarcoma, primary involvement of the uterine cervix is quite rare (Dehner et al., 2012). As somatic and germline genetic testing has become more common, cervical embryonal rhabdomyosarcoma (cERMS), ovarian Sertoli-Leydig cell tumor (SLCT) and multinodular goiter (MNG) have become linked to DICER1 syndrome, also known as pleuropulmonary blastoma (PPB) familial tumor predisposition and dysplasia syndrome. Because of the nascent understanding of this tumor predisposition syndrome, patients previously-diagnosed with a DICER1-associated tumor may not have been tested for genetic mutations, missing an opportunity for increased surveillance or genetic testing of family members. We present a patient with a second primary cERMS presenting synchronously with an ovarian SLCT. This case is unusual in that the patient had multiple primary lesions associated with DICER1 syndrome over a period of 12 years.
2. Case report
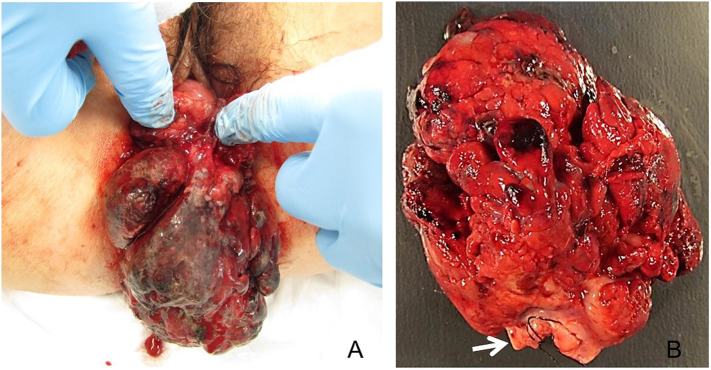
A 24-year-old G0P0 female was diagnosed with cERMS at age 17. She had been undergoing routine surveillance pelvic MRIs every six months to detect loco-regional recurrence when she was found to have a pelvic mass. Her previous history was significant for benign MNG which had been biopsied at age 12. At age 17, she experienced hemorrhage from a 7 × 10 × 6.5 cm cervical mass that was prolapsing through the vaginal introitus (Fig. 1A). She was treated surgically for the cervical mass with a partial trachelectomy (Fig. 1B). Histologic examination confirmed embryonal rhabdomyosarcoma with negative margins (Fig. 2). Cytogenic studies were negative for PAX3 or FOXO1 fusions that would be indicative of an alveolar component. Her staging evaluation included a normal bone marrow biopsy and bone scan, and otherwise unremarkable CT of the chest/abdomen/pelvis and MRI of the pelvis. She was classified as having Children's Oncology Group (COG) Group I, Stage 1 genitourinary rhabdomyosarcoma (Borinstein et al., 2018). Surgery was followed by combination chemotherapy with an intensified Ewing sarcoma regimen per the Children's Oncology Group study AEWS0031 (Womer et al., 2012) with interval compression VDC-IE (Vincristine, Doxorubicin, Cyclophosphamide, Ifosfamide and Etoposide). After adjuvant therapy, she underwent surveillance every six months with chest x-ray and abdominopelvic MRI for over six years, eventually transferring care to a local oncologist after she graduated college.
Fig. 1.
Large friable, hemorrhagic mass consistent with embryonal rhabdomyosarcoma protruding from the vaginal introitus (A) was excised with a partial trachelectomy (B). The mass was attached to the distal ectocervix (arrow).
Fig. 2.
Histologic evaluation of the cervical mass revealed a phyllodes-like growth pattern with characteristic subepithelial neoplastic stromal condensation (cambium layer) (A) and small islands of heterologous benign fetal cartilage (B). Immunohistochemical staining for muscle marker desmin (C) and skeletal muscle marker myogenin (D) confirmed the diagnosis of rhabdomyosarcoma.
Eleven months prior to her second diagnosis, she began to have intermenstrual spotting. A cervical polyp was removed in the office and the pathology was consistent with a benign fibroepithelial polyp. A routine pelvic MRI two months later showed a 2.5 cm cystic left adnexal mass that was characterized as a functional ovarian cyst. She denied any androgenic symptoms.
A repeat pelvic MRI six months later demonstrated that the left adnexal mass had increased to 7.3 cm with a 4.5 cm irregular solid component. Pelvic examination showed a new 2 × 5 cm cervical polyp which was resected in the office. Histology revealed an embryonal rhabdomyosarcoma at the distal tip of the polyp, confirmed by focal desmin and myogenin staining. Additional staging evaluation did not demonstrate any metastatic lesions.
After extensive counseling, the patient underwent a total laparoscopic hysterectomy with bilateral salpingectomy and a left oophorectomy. Her right ovary appeared normal and was transposed above her pelvic brim to avoid damage from future pelvic radiation therapy, if needed. The hysterectomy specimen revealed no residual rhabdomyosarcoma. The ovarian mass was a SLCT with intermediate differentiation, FIGO stage IA (Fig. 3). Spindle cell elements in the tumor stained negatively for desmin and myogenin, and somatic testing (OncoPanel - Brigham and Women's Hospital molecular diagnostics laboratory) showed two DICER1 mutations (Table 1). She recovered well from surgery and was referred to endocrinology for re-evaluation of her thyroid nodules.
Fig. 3.
The left oophorectomy specimen contained a large complex, nodular cystic mass (A). Histologic evaluation confirmed a Sertoli-Leydig cell tumor with intermediate differentiation, characterized by anastomosing chords of Sertoli cells with few intermixed Leydig cells, and foci of heterologous mucinous intestinal-type glands (bottom) (B).
Table 1.
Genetic mutation profiles.
| Original cERMS Somatic Testing | Second cERMS Somatic Testinga | Sertoli Leydig Cell Tumor Somatic Testing | Patient Germline Testing |
|---|---|---|---|
| DICER1 | DICER1 | DICER1 | DICER1 |
| c.904-1G > C (splice acceptor) | c.904-1G > C (splice acceptor) | c.904-1G > C (splice acceptor) | c.904-1G > C (splice acceptor) |
| c.5425G > A (p.G1809R) | c.5439G > A (p.E1813N) | c.5439G > A (p.E1813D) | |
|
BCOR c.3339del (p.S1113Rfs^46) |
|||
|
BRCA2 c.2348 T > C (p.V783A)b |
BRCA2 c.2348 T > C (p.V783A)⁎ |
BRCA2 c.2348 T > C (p.V783A)⁎ |
|
|
PALB2 c.2978C > T (p.T993 M)b |
PALB2 c.2978C > T (p.T993 M)⁎ |
PALB2 c.2978C > T (p.T993 M)⁎ |
|
|
MLH1 c.2978C > T (p.T993 M)b |
MLH1 c.2978C > T (p.T993 M)⁎ |
cERMS, cervical embryonal rhabdomyosarcoma.
Somatic testing for DICER1 mutation only due to small sample size requiring microdissection.
Variant of unknown significance.
After her recurrence, somatic mutation testing was performed on her original trachelectomy specimen (OncoPlus Large Tumor Universal Cancer Mutation Analysis Panel - University of Chicago Labs) and compared to DICER1 mutation testing from the recent polypectomy specimen (ResourcePath, Sterling, VA) (Table 1). The original tumor showed two pathogenic DICER1 mutations, a pathogenic BCOR mutation and variants of unknown significance (VUS) in BRCA2, PALB2 and MLH1. The new polyp had one DICER1 mutation in common, which was presumed to be germline, and a second unique pathogenic mutation, confirming a second primary tumor and not a late recurrence of her primary disease.
Due to the findings on somatic mutation testing, the patient was referred for genetic counseling and testing. Her three-generation pedigree was significant for a brother with benign thyroid biopsy at age 13, her mother had a goiter diagnosed in her twenties, and her maternal aunt had Hashimoto's disease diagnosed in her twenties. A targeted sequence analysis germline genetic test was performed on the patient (Invitae, San Francisco, CA) and identified the pathogenic DICER1 mutation common to both cERMS and the SLCT, in addition to the same VUS in BRCA2, PALB2 and MLH1 (Table1). Her family members were encouraged to seek genetic mutation testing.
3. Discussion
DICER1 syndrome is a relatively new clinical entity where the full repertoire of associated tumors has likely not yet been fully elucidated. DICER1 codes for an RNase IIIb endoribonuclease which processes immature microRNA (miRNA) (de Kock et al., 2017; Foulkes et al., 2014). Our patient harbored two DICER1 mutations in each of her cervical rhabdomyosarcoma tumors, but only one germline mutation. This is a typical pattern in DICER1-associated cancers where a second somatic mutation is found, a so-called “hotspot” mutation of the metal-binding domain (Witkowski et al., 2013; Witkowski et al., 2016).
The pathogenic role of DICER1 mutations was originally uncovered and linked to PBB by Hill, et al. in 2009 using a family-based linkage study of four families in the International PBB Registry (Hill et al., 2009). Other clinical manifestations include cystic nephromas, SLCTs, medulloblastomas of the eye, pineoblastoma, MNG, differentiated papillary thyroid carcinoma, juvenile germ cell tumor, and embryonal rhabdomyosarcoma (van Engelen et al., 2018; Schultz et al., 2018). Our patient had three lesions arising in the thyroid, cervix and ovary.
The most frequent manifestation of DICER1 mutations may be thyroid issues. A 2017 study by Kahn, et al. estimates that 75% of females (and 16% of males) with DICER1 mutations will develop MNG or undergo thyroidectomy by the age of 40, and there is a 16–18-fold risk of differentiated thyroid carcinoma (Khan et al., 2017). In addition, the study found that MNG is diagnosed at a younger age than those without DICER1 mutations in this population. In this case, our patient was diagnosed at age 12, which, given the current state of knowledge, should trigger a genetics work-up, particularly given her family history. This patient's MNG diagnosis, however, was made prior to the recognition of the full spectrum of clinical findings in DICER1 syndrome.
Embryonal rhabdomyosarcoma of the uterine cervix has also been linked to DICER1 mutations, and is especially rare, with the literature limited to published case reports and series (Dehner et al., 2012; Daya and Scully, 1988). The tumor is histologically similar to vaginal embryonal rhabdomyosarcoma, demonstrating a cambium layer (Dehner et al., 2012). Vaginal botryoides tumors are often diagnosed in the first three years of life, while cERMS is diagnosed later in the adolescent period, is typically less aggressive, and may be amenable to conservative surgery such as trachelectomy (Dehner et al., 2012).
Genetic testing can have a tremendous impact on treatment decisions. Conventionally, this case would be assumed to be a late recurrence of her cERMS. However, testing identified a unique DICER1 somatic mutation profile in the second tumor, proving it to be a low-risk second primary tumor. Five year survival for recurrent rhabdomyosarcoma is only 17% with no definitive chemotherapy or radiation salvage regimens (Pappo et al., 1999). Conversely, low-risk embryonal rhabdomyosarcoma has a five year overall survival of 98% with dose-reduced vincristine, dactinomycin, and cyclophosphamide (VAC) (Walterhouse et al., 2014).
The finding of a pelvic mass on surveillance imaging in a known DICER1 syndrome patient should elicit concern for a SLCT, usually a rare tumor, but prevalent in this population. Two cERMS case series noted five SLCTs in a total of 27 patients; one of these patients had genetic testing and was found to have a DICER1 germline mutation (Dehner et al., 2012; Daya and Scully, 1988). Two series of intermediately- and poorly-differentiated SLCTs with a combined 67 cases identified 66 with DICER1 somatic mutation. Of the 52 who had germline mutation testing, 72.6% had DICER1 heterozygous loss-of-function (LOF) mutations and another 5.8% had mosaicism for LOF mutations (de Kock et al., 2017; Schultz et al., 2017). Patients with SLCT and DICER1 mutations have a more favorable prognosis compared to sporadic SLCT (p = .02) and have a trend towards diagnosis at a younger age (median 16 vs. 21 years, p = .15) (Schultz et al., 2017).
Pediatricians and gynecologists should be aware of DICER1 syndrome and its link to cERMS and SLCT. Other DICER1-associated findings in the patient or family should pique concern, particularly early-onset MNG which may be the most penetrant manifestation of the syndrome (Witkowski et al., 2016; Khan et al., 2017). Consensus recommendations for testing and screening have been released and will be evolving as additional evidence is gathered on this genetic syndrome (Schultz et al., 2018). Based on the current consensus recommendations, testing should be offered for patients with a DICER1-associated finding, and screening should include imaging of the abdomen and pelvis to identify renal or genital tract anomalies, in addition to chest imaging, neuroimaging and thyroid palpation and imaging (Schultz et al., 2018).
In conclusion, this patient highlights the early-onset features of DICER1 syndrome and the risk of second primary cancers, which should trigger a referral to genetic counseling and testing in order to institute surveillance in patients and family members.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Author's statement
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
Each author was actively involved in revisions of the report and approved the final version for submission. Beyond that, the individual contributions are listed below.
Matthew Cowan: organized the specialist opinions, data, figures and drafted the report.
Tara Suntum: pediatric oncologist provided input on management of a second primary cervical cancer vs recurrence and drafted a section of the report.
Andrea D. Olivas: pathologist who prepared the images for the report.
Melody Perpich: genetic counselor who has cared for the patient and her family, and shared her expertise on genetic testing recommendations and cancer screening implications.
Mark A. Applebaum: pediatric oncologist who co-managed the patient and provided input on management of a second primary cervical cancer vs. recurrence.
Ricardo R. Lastra: pathologist who gave input on pathologic diagnosis of a second cancer versus a recurrence.
S. Diane Yamada: provided gynecologic oncology care for the patient, recognized the educational value of publishing the case, and highlighted key teaching points.
Contributor Information
Matthew Cowan, Email: t.obg.mcowan@bsd.uchicago.edu.
Tara Suntum, Email: tara.suntum@uchospitals.edu.
Andrea D. Olivas, Email: andrea.olivas@uchospitals.edu.
Melody Perpich, Email: mperpich@peds.bsd.uchicago.edu.
Mark A. Applebaum, Email: mapplebaum@peds.bsd.uchicago.edu.
Ricardo R. Lastra, Email: ricardo.lastra@uchospitals.edu.
S.Diane Yamada, Email: sdyamada@bsd.uchicago.edu.
References
- Borinstein S.C., Steppan D., Hayashi M., Loeb D.M., Isakoff M.S., Binitie O. Consensus and controversies regarding the treatment of rhabdomyosarcoma. Pediatr. Blood Cancer. 2018;65 doi: 10.1002/pbc.26809. [DOI] [PubMed] [Google Scholar]
- Daya D.A., Scully R.E. Sarcoma botryoides of the uterine cervix in young women: a clinicopathological study of 13 cases. Gynecol. Oncol. 1988;29:290–304. doi: 10.1016/0090-8258(88)90228-4. [DOI] [PubMed] [Google Scholar]
- de Kock L., Terzic T., Mccluggage W.G., Stewart C.J., Shaw P., Foulkes W.D. DICER1 mutations are consistently present in moderately and poorly differentiated Sertoli-Leydig cell tumors. Am. J. Surg. Pathol. 2017;41:1178–1187. doi: 10.1097/PAS.0000000000000895. [DOI] [PubMed] [Google Scholar]
- Dehner L.P., Jarzembowski J.A., Hill D.A. Embryonal rhabdomyosarcoma of the uterine cervix: a report of 14 cases and a discussion of its unusual clinicopathological associations. Mod. Pathol. 2012;25:602–614. doi: 10.1038/modpathol.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes W.D., Priest J.R., Duchaine T.F. DICER1: mutations, microRNAs and mechanisms. Nat. Rev. Cancer. 2014;14:662–672. doi: 10.1038/nrc3802. [DOI] [PubMed] [Google Scholar]
- Hill D.A., Ivanovich J., Priest J.R., Gurnett C.A., Dehner L.P., Desruisseau D. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325:965. doi: 10.1126/science.1174334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N.E., Bauer A.J., Schultz K.A.P., Doros L., Decastro R.M., Ling A. Quantification of thyroid Cancer and multinodular goiter risk in the DICER1 syndrome: a family-based cohort study. J. Clin. Endocrinol. Metab. 2017;102:1614–1622. doi: 10.1210/jc.2016-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappo A.S., Anderson J.R., Crist W.M., Wharam M.D., Breitfeld P.P., Hawkins D. Survival after relapse in children and adolescents with rhabdomyosarcoma: a report from the intergroup rhabdomyosarcoma study group. J. Clin. Oncol. 1999;17:3487–3493. doi: 10.1200/JCO.1999.17.11.3487. [DOI] [PubMed] [Google Scholar]
- Schultz K.A.P., Harris A.K., Finch M., Dehner L.P., Brown J.B., Gershenson D.M. DICER1-related Sertoli-Leydig cell tumor and gynandroblastoma: clinical and genetic findings from the international ovarian and testicular stromal tumor registry. Gynecol. Oncol. 2017;147:521–527. doi: 10.1016/j.ygyno.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz K.A.P., Williams G.M., Kamihara J., Stewart D.R., Harris A.K., Bauer A.J. DICER1 and associated conditions: identification of at-risk individuals and recommended surveillance strategies. Clin. Cancer Res. 2018;24(10):2251–2261. doi: 10.1158/1078-0432.CCR-17-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Engelen K., Villani A., Wasserman J.D., Aronoff L., Greer M.C., Tijerin Bueno M. DICER1 syndrome: approach to testing and management at a large pediatric tertiary care center. Pediatr. Blood Cancer. 2018;65 doi: 10.1002/pbc.26720. [DOI] [PubMed] [Google Scholar]
- Walterhouse D.O., Pappo A.S., Meza J.L., Breneman J.C., Hayes-Jordan A.A., Parham D.M. Shorter-duration therapy using vincristine, dactinomycin, and lower-dose cyclophosphamide with or without radiotherapy for patients with newly diagnosed low-risk rhabdomyosarcoma: a report from the soft tissue sarcoma Committee of the Children's oncology group. J. Clin. Oncol. 2014;32:3547–3552. doi: 10.1200/JCO.2014.55.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski L., Mattina J., Schonberger S., Murray M.J., Choong C.S., Huntsman D.G. DICER1 hotspot mutations in non-epithelial gonadal tumours. Br. J. Cancer. 2013;109:2744–2750. doi: 10.1038/bjc.2013.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski L., Mccluggage W.G., Foulkes W.D. Recently characterized molecular events in uncommon gynaecological neoplasms and their clinical importance. Histopathology. 2016;69:903–913. doi: 10.1111/his.13058. [DOI] [PubMed] [Google Scholar]
- Womer R.B., West D.C., Krailo M.D., Dickman P.S., Pawel B.R., Grier H.E. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's oncology group. J. Clin. Oncol. 2012;30:4148–4154. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]