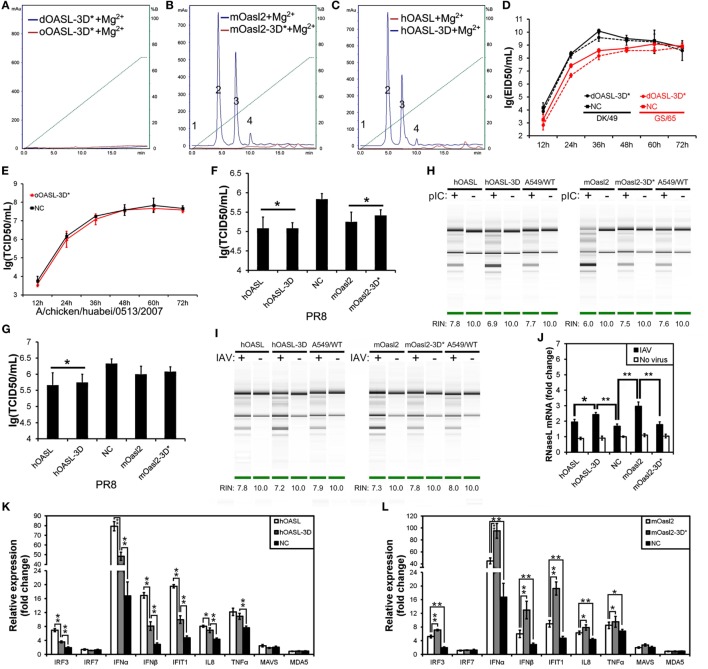
Figure 4.
Three conserved sites of duck, ostrich, human, and mouse OASL being homologous to D75-D77-D148 of human 2′–5′-oligoadenylate synthetase 1 are a button for switching on/off the OAS/RNase L pathway. The 2–5A synthetase reaction was treated with alkaline phosphatase and separated using a Mono Q column. Gene expression in cells were calculated relative mRNA level to that of GAPDH and presented as fold change against the corresponding of A549 cells expressing empty vector (NC) without PR8 virus infection (two-tailed Student’s t test, n = 3). The data are expressed as the mean ± SD. *P < 0.05; **P < 0.01. (A–C) The elution profiles produced by dOASL-3D* or oOASL-3D* (A), mOasl2 or mOasl2-3D* (B), and hOASL-3D or hOASL proteins (C). (D,E) dOASL-3D* and oOASL-3D* did not inhibit the DK/59 and GS/65 in DF1 cells (D) or CK/0513 (E) virus replication in DF1OASL−/− cells, whose RIG-I is naturally absent. (F,G) hOASL-3D and mOasl2 slightly or significantly inhibited the replication of PR8 [multiplicity of infection (MOI) = 0.001] virus in A549 (F) or HeLa (G) cells. (H,I) rRNA cleavage induced by hOASL-3D and mOasl2 in A549 cells stimulated with pIC (H) (500 ng/mL) or infected with PR8 virus (I) MOI = 1. (J–L) hOASL-3D and mOasl2 significantly increased the expression of RNase L (J) and six genes (K,L) related to IFN signaling upon infection with PR8 virus (MOI = 0.001).