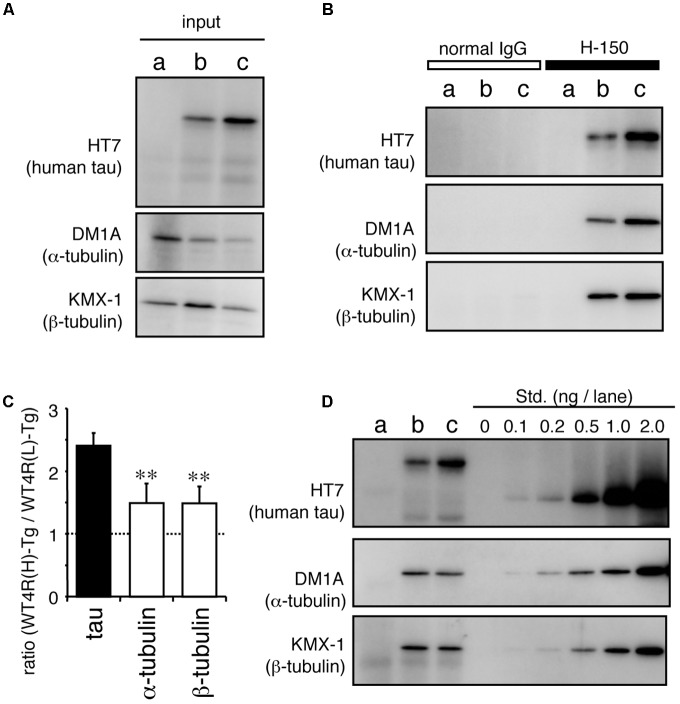
FIGURE 7.
MT-unbound tau in tau-Tg worms interacts with α/β-tubulin dimers in the soluble fraction. MT-unbound soluble fractions were prepared from Mock-Tg (a), WT4R(L)-Tg (b), and WT4R(H)-Tg (c) worms and subjected to the immunoprecipitation using anti-tau IgG (H-150). (A) Expression of tau, α-tubulin, and α-actin in the total lysate (input) are shown. (B) Immunoprecipitated proteins were analyzed using anti-tau (HT7), anti-α-tubulin (DM1A), and anti-β-tubulin (KMX-1) antibodies. (C) Relative amount of tau and α/β-tubulin in the co-immunoprecipitaed fraction from tau-Tg worms were quantified (mean ± SEM, n = 6). (D) Quantitative analysis of each protein indicates that the nearly equal amount of α- and β-tubulin were co-immunoprecipitated with tau. Std. indicates the purified recombinant tau (for HT7) or porcine tubulins (for DM1A and KMX-1). Statistical significance was analyzed by Tukey’s post hoc test (∗∗p < 0.01).