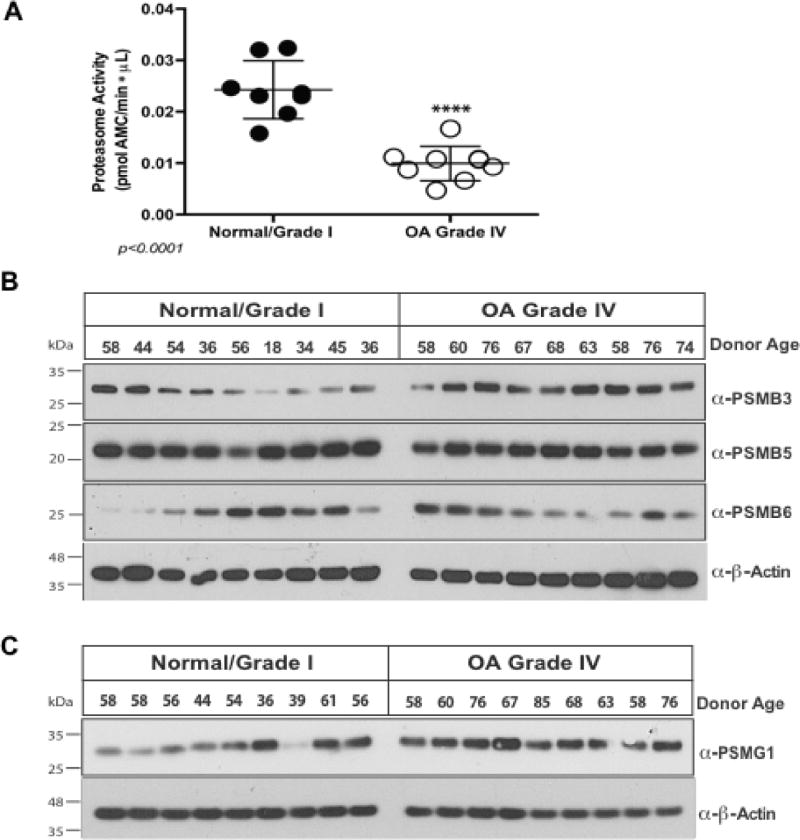
Figure 3. Proteasome activity is diminished in OA chondrocytes, without significant decrease in levels of critical 20S inner ring β subunits and proteasome assembly chaperone PSMG1.
(A) Lysates were collected from normal/grade I (n=8) and grade IV (n=8) chondrocytes, in the absence of protease inhibitors; 10 µg protein aliquots were assayed for 20S proteasome function, using SUC-LLVY-AMC substrate, measuring at 37°C at 351nm/430nm excitation/emission. Replicate controls had proteasome inhibitor MG132 (200 µM). We observed greater than 50% decrease (p<0.0001, t-test) in proteasome protease activity in OA. (B) Aliquots (10 µg) of protein extracted from primary chondrocytes (n=18 donors, 9 normal and 9 grade IV) were analyzed by Western blot to determine levels of critical 20S proteasomal inner ring β-subunits, revealing no significant variation between normal and grade IV. (C) PSMG1 was not appreciably decreased in a panel of OA chondrocytes compared to normal donor cells.