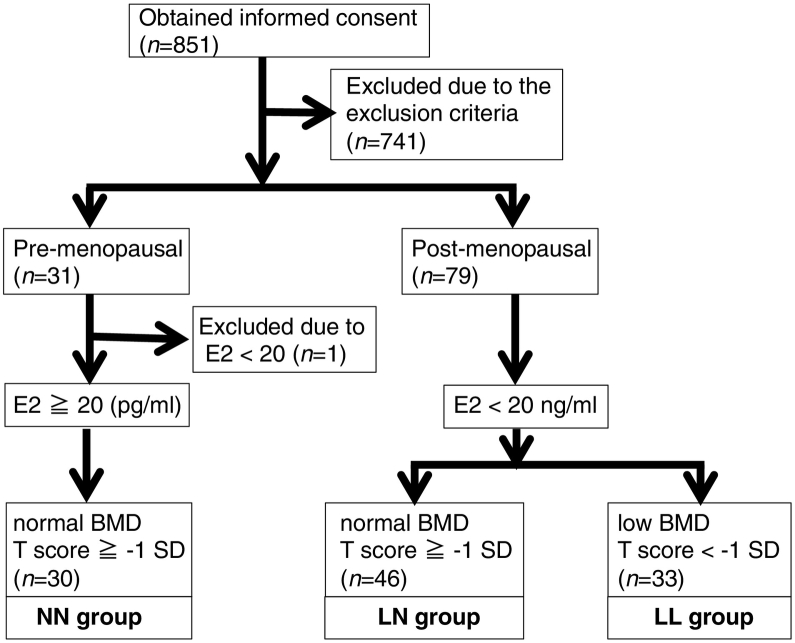
Fig. 1.
Selection of study subjects.
Written informed consent was obtained from 851 women in our hospital, and 741 subjects were excluded based on indicated criteria. Thus, the number of enrolled subjects was: normal estradiol and normal BMD (NN) group, 30; low estradiol with normal BMD (NL) group, 46; and low estradiol with low BMD (LL) group, 33. Exclusion criteria included drug usage (not limited to anti-osteoporosis drugs), unstable menstruation status, lean or obese status (BMI < 18.5 or BMD > 30, respectively) and incomplete data sets. BMI, body mass index; E2, estradiol; BMD, bone mineral density.