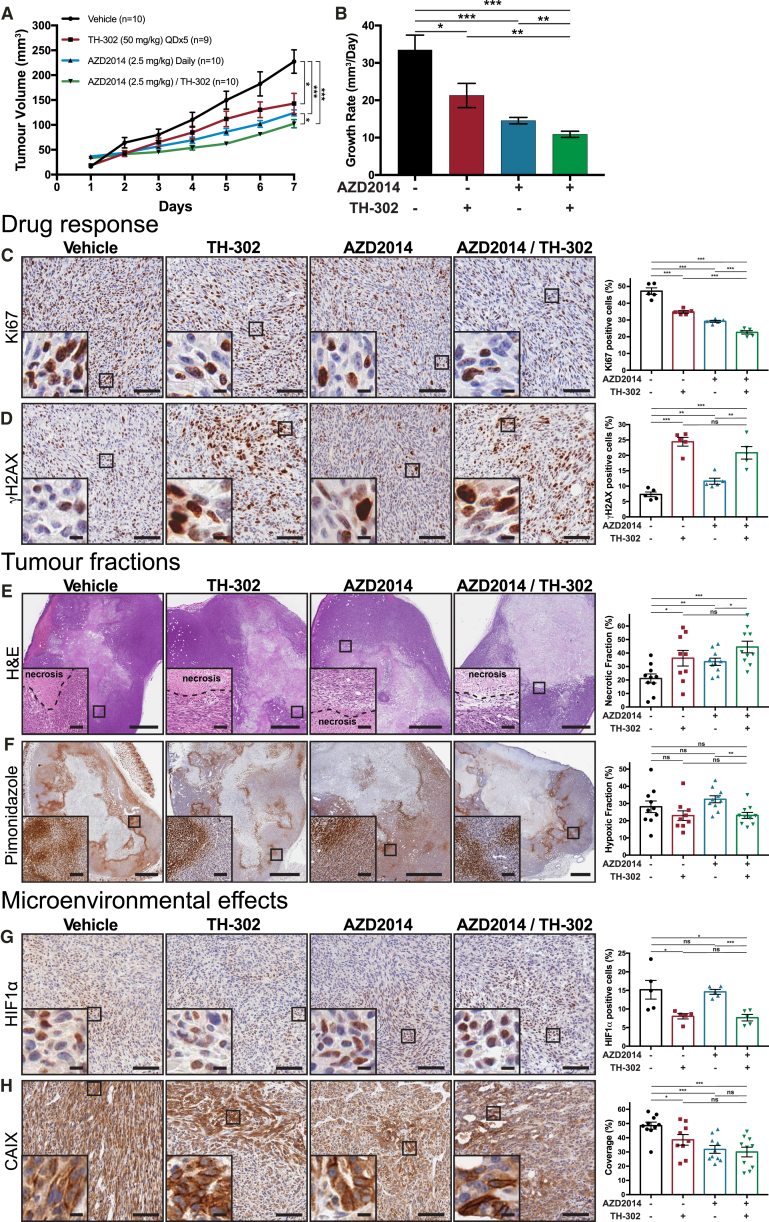
Figure 3.
Assessment of KPC Subcutaneous Xenografts Treated with TH-302 (50 mg/kg) and AZD2014 (2.5 mg/kg)
(A and B) Tumor volume measurements (A) and average linear growth rate (B) of tumors over 7 days from vehicle/saline (n = 10), vehicle/TH-302 (n = 9), AZD2014/saline (n = 10), and AZD2014/TH-302 (n = 10).
(C and D) Immunohistochemistry (IHC) staining of drug response in tumors assessed for the proliferative marker Ki67 (n = 5 tumors/treatment) (C) and DNA damage response (γH2AX, n = 5 tumors/treatment) (D). Scale bars, 100 μm; insets, 10 μm. Mean ± SEM.
(E and F) Staining for the necrotic (using H&E) (E) and hypoxic (pimonidazole IHC) (F) tumor fractions from vehicle/saline (n = 10), vehicle/TH-302 (n = 9), AZD2014/saline (n = 10), and AZD2014/TH-302 (n = 10) treatments. Scale bars, 1 mm; insets, 100 μm. Mean ± SEM.
(G) HIF1α (n = 5 tumors/treatment) IHC staining of tumors. Scale bars, 100 μm; insets, 10 μm. Mean ± SEM.
(H) Carbonic anhydrase IX (CAIX) IHC staining of tumors from vehicle/saline (n = 10), vehicle/TH-302 (n = 9), AZD2014/saline (n = 10), and AZD2014/TH-302 (n = 10) treatments. Scale bars, 100 μm; insets, 10 μm. Mean ± SEM. p values are from a Student two-tailed parametric t test in all panels.
∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. See also Figure S3.