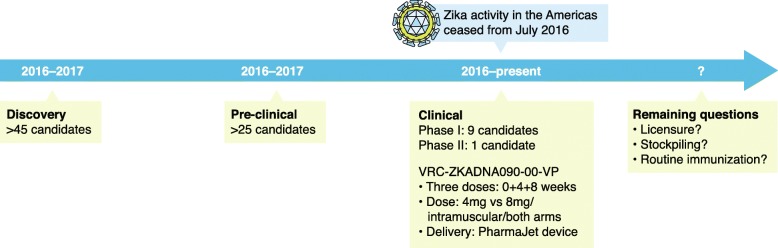
Fig. 1.
Zika vaccine development pathway. The vaccine development pathway starts with basic science/discovery and a lead candidate vaccine undergoes preclinical evaluation for safety and immunogenicity in animal models where high-quality data are needed to justify to a regulatory agency (e.g., US Food and Drug Administration (FDA) or European Medicines Agency (EMA)) that a vaccine candidate is suitable to be evaluated in clinical trials. Following successful clinical trials, a vaccine will be licensed for use