Highlights
-
•
While endosalpingiosis is often asymptomatic & incidental, florid cystic endosalpingiosis can have a variable presentation
-
•
Cystic endosalpingiosis can be difficult to differentiate from other non-neoplastic peritoneal inclusion cysts
-
•
Although associated with serous pelvic neoplasms, there is no evidence for oophorectomy at the completion of fertility
-
•
There is no strong evidence that hysterectomy along with cyst resection leads to improved outcomes if pathology is benign
-
•
This is the first reported case of successful assisted-reproductive therapy after resected florid cystic endosalpingiosis
1. Introduction
Endosalpingiosis is a benign condition characterized by the presence of ectopic, cystic glands lined with ciliated epithelium similar to that found in the fallopian tube (Clement and Young 1999). It is most commonly found on pelvic organs such as the uterine serosa, fallopian tube serosa, ovaries, round ligament, and bladder, but has been documented in many other abdominal peritoneal and subperitoneal surfaces, including in retroperitoneal lymph nodes (Burmeister et al. 1969; Cajigas and Axiotis 1990, and Chen 1981). It was first described by Sampson in 1930 when epithelium resembling the fallopian tube was noted in the pelvises of women who had previously undergone salpingectomies or tubal sterilization (Prentice et al. 2012). While endosalpingiosis can have a similar appearance to endometriosis, usually yellow-white punctate cystic lesions, it differs in that there is no endometrial stroma noted and it does not elicit the inflammatory response associated with pelvic pain and infertility (deHoop et al. 1997). However, the pathogenesis of both diseases are similar in that they results from műllerianosis, where either multipotent peritoneal mesothelium undergoes metaplasia into oviduct epithelium or developed műllerian tissue is ectopically displaced (Ong et al. 2004, and Batt and Yeh 2013). While endosalpingiosis is often asymptomatic and an incidental intraoperative finding, florid cystic endosalpingiosis usually presents clinically with pelvic pain, a mass noted on physical examination, or multiple cystic tumors seen on imaging and is very rare (Clement and Young 1999).
2. Case presentation
A 43-year-old nulligravid woman initially presented to her local gynecologist for left sided pelvic pain progressing in severity. MRI of the pelvis demonstrated a 14 cm by 7 cm multicystic left adnexal mass, and numerous cystic masses on the anterior and fundal surfaces of the uterus [Fig. 1A]. Her past medical history was significant for primary infertility and class III obesity with a BMI of 45 kg/m2. Her past surgical history included a laparoscopy 22 years prior for a benign ovarian mass, with no report of multiple cystic masses present at that time. A CA-125 tumor marker at the time of presentation was 30.1 U/mL, suggesting benign disease. She underwent an operative laparoscopy with left ovarian cystectomy. Numerous cystic lesions of various sizes emanating from the uterine surface measuring <1 cm to 3 cm were noted. Only the largest, approximately 7 to 8 cm, was excised from the uterine serosa. By report, pathology was consistent with a serous cystadenoma, but endosalpingiosis was also considered. Pelvic washing and cytologic evaluation of cyst aspirate were negative for malignancy. Hysterectomy was recommended; however, the patient sought a second opinion due to desire for fertility via uterine preservation and donor eggs. The decision was made to proceed with a robotic exploration and excision of cysts from the uterine serosa, bladder, and ovaries with the goal of uterine preservation.
Fig. 1.
Preoperative and postoperative MRI findings. A) Preoperative MRI shows multiple T2-enhancing cystic lesions along the uterine serosa and adnexa. B) Six weeks after surgery there is no evidence of recurrent disease.
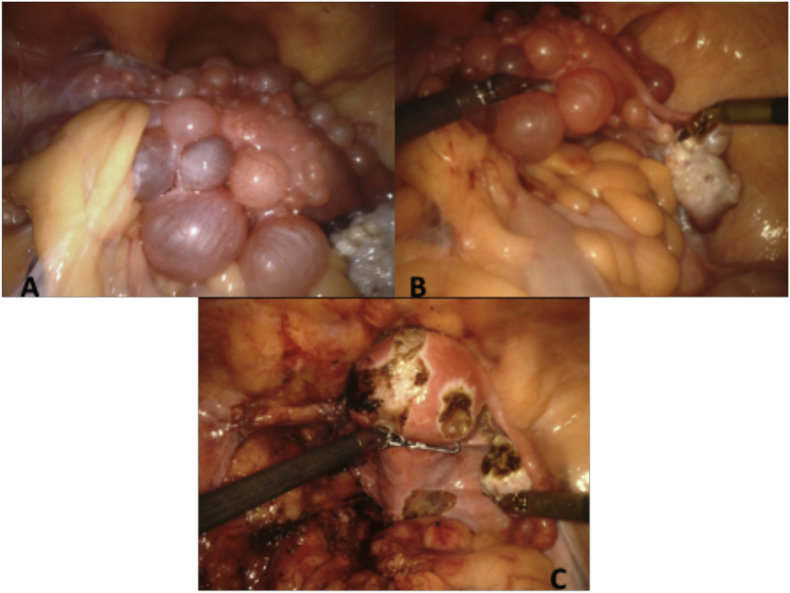
In the operating room upon abdominal entry, the liver, diaphragm, bowel, and omental surfaces were unremarkable. Multiple simple-appearing cysts arising directly from the uterine serosa and pelvic peritoneum were visualized [Fig. 2A, B]. The left ovary was significantly enlarged with innumerable ovarian cysts, and was adhered to the left pelvic sidewall and sigmoid colon mesentery. The left fallopian tube had a paratubal cyst. The right ovary had several smaller ovarian cysts, and the right fallopian tube was normal in appearance. There were also multiple cysts noted on the overlaying pelvic peritoneum. A left salpingo-oophorectomy was performed given the extensive involvement, including cysts extending into the broad ligament and underneath the round ligament. Cysts were then resected from the right ovary and the affected visceral pelvic peritoneum. The uterine corpus was normal in appearance at the conclusion of the procedure with minimal impact on the myometrium [Fig. 2C]. Pathology review classified the process as extensive cystic endosalpingiosis involving left and right ovaries and uterine serosa rather than serous cystadenoma due to absence of associated ovarian stroma or fibromatous stroma [Fig. 3A, B].
Fig. 2.
Intraoperative findings: A) Intraoperative examination of the pelvis. Significant adhesions around the left ovary were seen, along with cystic lesions covering the uterine serosa. B) Highlight of cystic lesions along the uterus and right ovary. C) Uterus, left round ligament, right fallopian tube, and right ovary following completion of left salpingo-oophorectomy and removal of cysts.
Fig. 3.
Numerous simple cystic structures involved the uterine serosa and outer myometrium. A) A single layer of ciliated columnar cells lined the cysts without associated ovarian or fibromatous stroma. B) Bundles of myometrial smooth muscle are seen adjacent to epithelium.
The patient's postoperative course was unremarkable and she discharged on postoperative day one. A follow-up MRI was recommended in 3 months, which demonstrated no evidence of recurrent disease [Fig. 1B]. The patient resumed pursuits at fertility with the utilization of donor eggs, and successfully carried a dichorionic diamniotic twin pregnancy to term. She delivered a healthy baby boy and girl via Cesarean at 38 weeks following an uncomplicated pregnancy.
3. Discussion
While endosalpingiosis is often asymptomatic and incidental, florid cystic endosalpingiosis can have a variable presentation. Both pathologies can present with pelvic pain. One retrospective study of 51 laparoscopies for pelvic pain found six cases of endosalpingiosis in locations associated with the patient's pelvic pain (Keltz et al. 1995). All six patients also obtained relief after surgery, which suggests a causal relationship. However, endosalpingiosis often coexists with endometriosis, and the majority of surgeons treat by ablating the lesions rather than obtaining a histologic diagnosis (Ong et al. 2004). Additional studies confirming this association are limited.
Cystic endosalpingiosis can be difficult to differentiate from other non-neoplastic peritoneal inclusion cysts, which should also be included in the differential diagnosis (Baker et al. 2014). While endosalpingiosis is thought to originate from ectopic or metaplastic műllerian tissue, multilocular peritoneal inclusion cysts are mesothelial.
Endosalpingiosis is often found concurrently with other serous pelvic neoplasms, which include peritoneal serous borderline tumors, low-grade serous primary peritoneal carcinomas, and high-grade serous primary peritoneal carcinomas (Baker et al. 2014). Prentice et al. (2012) found that women with borderline ovarian tumors had concurrent endosalpingiosis on the ovary in 37% of patients, on the fallopian tube in 21%, on the omentum in 9%, on the uterine serosa in 7%, as well as on other sites which included the uterovesical reflection, lymph nodes, cervix, bladder, peritoneum, and appendix. Another large series of endosalpingiosis cases by Esselen et al. (2016) included 838 patients reported an increased odds of serous borderline tumors in patients with endosalpingiosis (odds ratio [OR] 10.2, 95% CI 5.1-20.7) as compared to a population control group of ovarian cancer cases. There has also been suggestion of an increased odds of clear cell and invasive mucinous tumors (Esselen and Barbieri, 2017). Despite this association, there is no evidence supporting oophorectomy at the completion of fertility in patients with known endosalpingiosis.
In our patient described above, surgical removal of the cystic neoplasms was pursued with the goal of fertility preservation. Thorough inspection of the pelvic organs is recommended when endosalpingiosis is seen intraoperatively and, given the association described above and the difficulty differentiating florid cystic endosalpingiosis from other cystic pelvic neoplasms by clinical features and gross examination, removal for histologic evaluation is necessary to rule out tumors of malignant potential. However, particularly if frozen pathology yields benign disease, there is no strong evidence to indicate that a hysterectomy would lead to improved outcomes.
To date, there are no reported cases of patients with resected florid cystic endosalpingiosis undergoing successful assisted reproductive therapy following surgery. There is no proven association between endosalpingiosis and infertility, and patients with the diagnosis that do not have concomitant endometriosis are usually treated as unexplained infertility (Esselen and Barbieri 2017). Our patient was able to undergo in vitro fertilization and achieve a dichorionic diamniotic twin pregnancy following surgery with fertility preservation. This supports the practice of removing the lesions associated with florid cystic endosalpingiosis, but retaining the uterus and ovaries when the pathology is benign and fertility is desired.
4. Conclusion
In conclusion, we present a case of florid cystic endosalpingiosis in a patient that desired fertility preservation. Based on a review of the literature, although there is an association of endosalpingiosis with serous pelvic neoplasms, in the absence of suspicion for malignancy there is no evidence to suggest hysterectomy and oophorectomy is necessary.
Conflict of interest statement
The authors have no conflicts of interest.
Informed consent
Written informed consent was obtained from the patient and can be provided upon request.
References
- Baker P.M., Clement P.B., Young R.H. Selected topics in peritoneal pathology. Int. J. Gynecol. Pathol. 2014;33:393. doi: 10.1097/PGP.0000000000000146. [DOI] [PubMed] [Google Scholar]
- Batt R.E., Yeh J. Müllerianosis: four developmental (embryonic) mullerian diseases. Reprod. Sci. 2013;20(9):1030–1037. doi: 10.1177/1933719112472736. (PubMed PMID: 23314961) [DOI] [PubMed] [Google Scholar]
- Burmeister R.E., Fechner R.E., Franklin R.R. Endosalpingiosis of the peritoneum. Obstet. Gynecol. 1969;34:310–318. (PubMed PMID 5805528) [PubMed] [Google Scholar]
- Cajigas A., Axiotis C.A. Endosalpingiosis of the vermiform appendix. Int. J. Gynecol. Pathol. 1990;9:291–295. doi: 10.1097/00004347-199007000-00009. (PubMed PMID: 2373590) [DOI] [PubMed] [Google Scholar]
- Chen K.T. Benign glandular inclusions of the peritoneum and periaortic lymph nodes. Diagn. Gynecol. Obstet. 1981;3:265–268. (PubMed PMID: 7327079) [PubMed] [Google Scholar]
- Clement P.B., Young R.H. Florid cystic endosalpingiosis with tumor-like manifestations: a report of four cases including the first reported cases of transmural endosalpingiosis of the uterus. Am. J. Surg. Pathol. 1999;23(2):166–175. doi: 10.1097/00000478-199902000-00005. (PubMed PMID: 9989843) [DOI] [PubMed] [Google Scholar]
- deHoop T.A., Mira J., Thomas M.A. Endosalpingiosis and chronic pelvic pain. J. Reprod. Med. 1997;42(10):613–616. (PubMed PMID: 9350013) [PubMed] [Google Scholar]
- Esselen, K., Barbieri, R., 2017. Endosalpingiosis. Up to date. https://www.uptodate.com/ contents/endosalpingiosis (Accessed Feburary 7, 2018).
- Esselen K.M., Terry K.L., Samuel A., Elias K.M., Davis M., Welch W.R., Muto M.G., Ng S.W., Berkowitz R.S. Endosalpingiosis: more than just an incidental finding at the time of gynecologic surgery? Gynecol. Oncol. 2016;142(2):255–260. doi: 10.1016/j.ygyno.2016.05.036. (PubMed PMID: 27261327) [DOI] [PubMed] [Google Scholar]
- Keltz M.D., Kliman H.J., Arici A.M., Olive D.L. Endosalpingiosis found at laparoscopy for chronic pelvic pain. Fertil. Steril. 1995;64:482–485. (PubMed PMID: 7641898) [PubMed] [Google Scholar]
- Ong N.C.S., Maher P.J., Pyman J.M., Readman E., Gordon S. Endosalpingiosis: more than just an incidental finding at the time of gynecologic surgery? Gynecol. Surg. 2004;1:11. [Google Scholar]
- Prentice L., Stewart A., Mohiuddin S., Johnson N.P. What is endosalpingiosis? Fertil. Steril. 2012;98(4):942–947. doi: 10.1016/j.fertnstert.2012.06.039. (PubMed PMID: 22819185) [DOI] [PubMed] [Google Scholar]