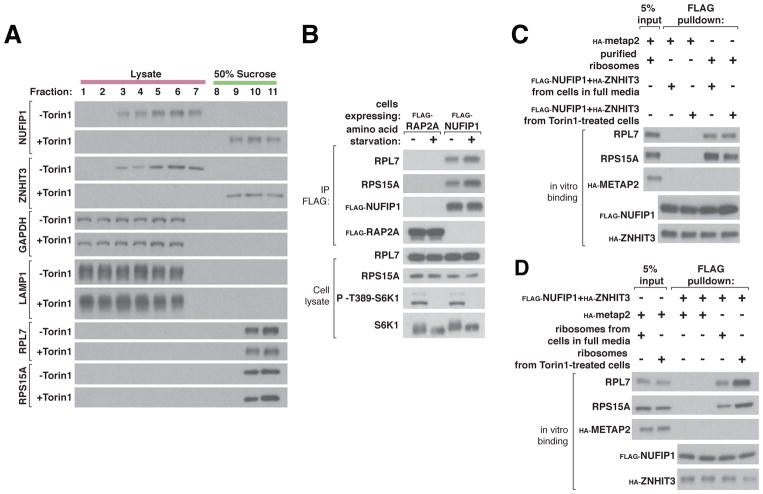
Fig. 3. NUFIP1-ZNHIT3 interacts with ribosomes in an mTORC1-dependent fashion.
(A) mTOR inhibition increases the amount of NUFIP1-ZNHIT3 that co-migrates with ribosomes. HEK-293T cell lysates prepared from cells in full media or treated with 250 nM Torin1 were fractionated over a 50% sucrose cushion. Fractions were collected and the indicated proteins analyzed by immunoblotting.
(B) Amino acid deprivation increases the amount of ribosomes that co-immunoprecipitates with NUFIP1. HEK-293T cells stably expressing FLAG-NUFIP1 were deprived of amino acid for 1 hour. Lysates and FLAG immunoprecipitates were prepared and analyzed for the indicated proteins by immunoblotting. FLAG-Rap2A was used as a negative control.
(C) In vitro, purified NUFIP1-ZNHIT3 binds to ribosomes and the interaction is not affected by whether or not NUFIP1-ZNHIT3 was obtained from cells with inhibited mTOR. The FLAG-NUFIP1-HA-ZNHIT3 complex was purified from HEK-293T cells in full media or treated with 250 nM Torin1 for 1 hour and immobilized on a FLAG affinity resin. Equal amounts of ribosomes obtained from cells in full media were added to the immobilized FLAG-NUFIP1-HA-ZNHIT3 complex and the proteins captured analyzed by immunoblotting. Ribosomes were purified as described in the supplementary materials. Purified HA-METAP was used as a negative control.
(D) In vitro, ribosomes purified from cells with mTOR inhibition bind better to NUFIP1-ZNHIT3 than those from cells in full media. Ribosomes were purified from HEK-293T cells in full media conditions or treated with 250 nM Torin1 for 1 hour. The FLAG-NUFIP1-HA-ZNHIT3 complex was immobilized on FLAG affinity beads and equal amounts of ribosomes were added. Proteins captured by the FLAG affinity beads were analyzed by immunoblotting. HA-METAP2 served as a negative control.