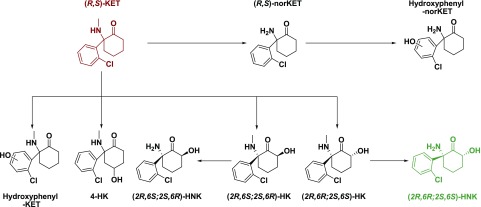
Fig. 2.
Minor metabolic pathways. Although the majority of ketamine is metabolized via the major metabolic pathways (Fig. 1), there are several minor metabolic pathways, which provide unique, albeit low abundance, ketamine metabolites. The aryl ring of ketamine can be directly hydroxylated by flavin-containing mono-oxygenase enzymes or CYP2C9 to provide hydroxyphenyl-ketamine (hydroxyphenyl-KET). 4-Hydroxyketamine has also been observed; however, the metabolic enzymes responsible for this are currently unknown. CYP3A5 can directly hydroxylate ketamine at the six position to provide (2R,6S;2S,6R)-HK. Demethylation of (2R,6S;2S,6R)-HK with CYP3A5 provides (2R,6S;2S,6R)-HNK. CYP2A6 can also directly hydroxylate ketamine to provide (2R,6R;2S,6S)-HK, which is then transformed to (2R,6R;2S,6S)-HNK. Finally, norketamine can be hydroxylated via an unknown enzyme directly on the aryl rich to provide hydroxyphenyl-norketamine (hydroxyphenyl-norKET).