Fig. 1.
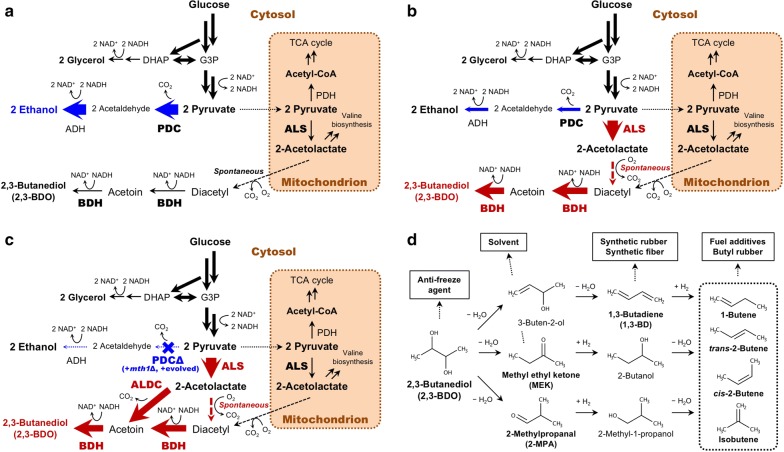
Pyruvate carbon flux tugging strategies for increased 2,3-butanediol (2,3-BDO) production and reduced ethanol subgeneration in yeast. a Ethanol and 2,3-BDO biosynthetic pathways in the wild-type yeast S. cerevisiae. Yeast preferentially produces ethanol even under aerobic conditions via the “Crabtree effect”, which remains to be completely elucidated. However, the strong activities of the glycolytic Embden–Meyerhof–Parnas (EMP) pathway and fermentative ethanol biosynthetic pathway in maintaining the redox homeostasis of NADH likely contribute to the Crabtree effect. Wild-type S. cerevisiae produces a small amount of 2,3-BDO via 2-acetolactate synthesized in the mitochondria. b Metabolic engineering strategy to tug the pyruvate carbon flux in yeast. High-activity cytosolic (or mitochondrial) acetolactate synthase (ALS) is required for increased 2,3-BDO production and reduced ethanol subgeneration. c A pyruvate decarboxylase (PDC)-deficient yeast (PDCΔ) strain (containing the MTH1-ΔT allele and subjected to laboratory evolution) was used to further ensure tugging of the pyruvate carbon flux and secure higher 2,3-BDO production. Acetolactate decarboxylase (ALDC) and butanediol dehydrogenase (BDH) were additionally (over)expressed to avoid clogging the carbon flux toward 2,3-BDO biosynthesis. d Applications of 2,3-BDO and its derivatives. 2,3-BDO can be chemically converted to various chemicals, including synthetic rubbers and fuel additives. G3P glyceraldehyde 3-phosphate, DHAP dihydroxyacetone phosphate, TCA tricarboxylic acid, ADH alcohol dehydrogenase, PDH pyruvate dehydrogenase