Figure 3.
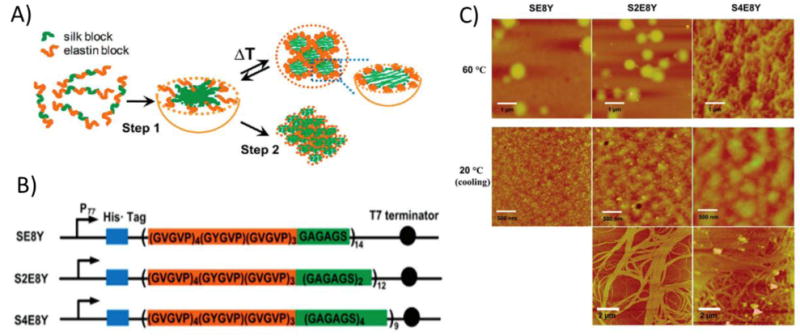
Silk-like and elastin-like polypeptide fusion proteins (SLP-ELP) exhibited a temperature-sensitive two step assembly process into coacervates, gels, and fibers. A) Scheme showing micelle assembly of SLP-ELP protein. Upon mixing, proteins self-assembled into micelles with hydrophobic SLPs in the core and ELPs in the shell. Upon heating above the lower critical solution temperature (LCST), the ELP block underwent hydrophobic collapse and aggregated with other ELP blocks in neighboring micelles. B) Sequence of ELP-SLP proteins. The frequency of the ELP guest residue was 1/8 tyrosine and 7/8 valine. The ratio of SLP to ELP varied between 1:8 (SE8Y), 2:8 (S2E8Y), and 4:8 (S4E8Y). C) Atomic force microscopy (AFM) images of aggregates and fibers formed by SLP-ELP proteins. When heated, the proteins formed small aggregates. Upon cooling, proteins with higher ratios of SLP to ELP formed fibrous networks. Reprinted (adapted) with permission from Biomacromolecules, Tunable Self-Assembly of Genetically Engineered Silk-Elastin-like Protein Polymers, 12, 2011, Xiao-Xia Xia, Qiaobing Xu, Xiao Hu, Guokui Qin, David L. Kaplan. Copyright 2011 American Chemical Society.
