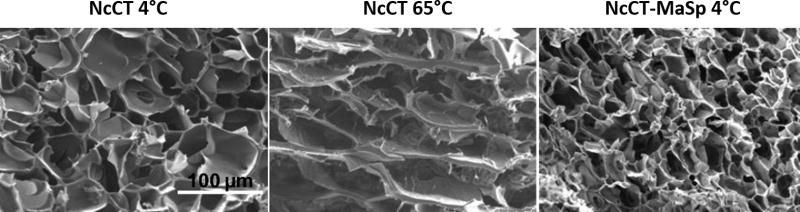
Figure 4.
Temperature-triggered formation of gels by silk-like polypeptides at both low and high temperature. Scanning electron microscopy (SEM) images of hydrogels formed by proteins from the C-terminal domain of N. clavipes major ampullate spidroin 1 (NcCT) and the fusion proteins containing NcCT and repeated sequences from the central region of major ampullate spidroin (NcCT-MaSP). NcCT proteins, which do not contain repetitive sequences, formed irreversible hydrogels with sheet-like structures when crosslinked at high temperatures. Both proteins formed porous hydrogel networks when crosslinked at low temperatures. Reprinted (adapted) with permission from Biomacromolecules, Dual Thermosensitive Hydrogels Assembled from the Conserved C-Terminal Domain of Spider Dragline Silk, 16, 2015, Zhi-Gang Qian, Ming-Liang Zhou, Wen-Wen Song, Xiao-Xia Xia. Copyright 2015 American Chemical Society.