Figure 5.
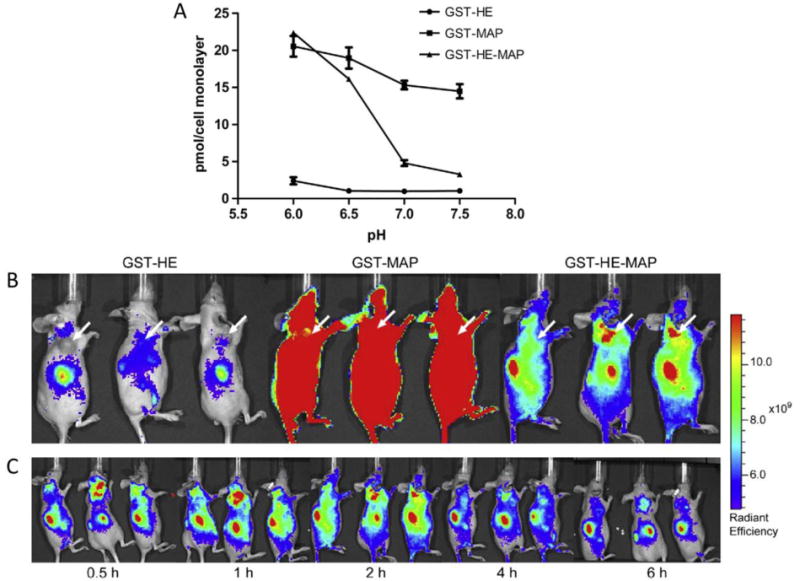
A cell penetrating peptide, named model amphipathic peptide (MAP), delivered a model drug, glutathione S-transferase (GST), in slightly acidic conditions in vivo because of its fusion with a histidine- and glutamic acid-containing peptide (HE). A) HeLa cell association of GST-carrying proteins. GST-HE-MAP was pH-sensitive and associated more strongly at pH <7, whereas GST-MAP or GST-HE were not pH sensitive. B) Fluorescently-labeled localization of GST-carrying proteins in a tumor model mouse system. GST-HE-MAP localized to tumor cells, whereas GST-MAP associated indiscriminately and GST-HE had very low association. C) Time monitoring of GST-MAP-HE protein in mouse after injection. GST-MAP-HE localized to the tumor site in less than 30 minutes. Reprinted from Biomaterials, 35/13, Likun Fei, Li-Peng Yap, Peter S. Conti, Wei-Chiang Shen, Jennica L. Zaro, Tumor targeting of a cell penetrating peptide by fusing with a pH-sensitive histidine-glutamate co-oligopeptide, 4082–4087, Copyright 2014, with permission from Elsevier.
