Figure 9.
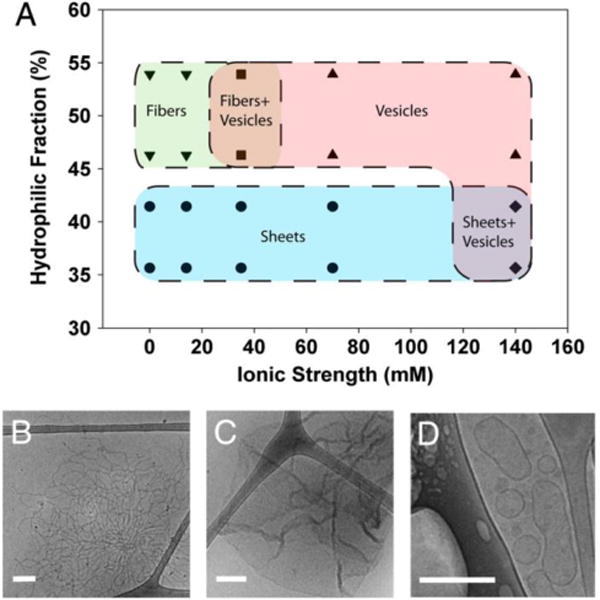
Self-assembly structures determined by protein hydrophilicity and ionic strength in solution. A) Structures of self-assembled oleosins. Proteins with a lower hydrophilic fraction favored structures with smaller curvature (e.g., sheets). For proteins with the same hydrophilic fraction, higher ionic strength in the solution favored vesicle formation. Data points represent actual experimental observations. B-D) Representative cryo-TEM images of fibers, sheets, and vesicles, respectively. Reprinted with permission from Proceedings of the National Academy of Sciences of the United States of America, Self-assembly of tunable protein suprastructures from recombinant oleosin, 109, 2012, Kevin B. Vargo, Ranganath Parthasarathy, Daniel A. Hammer. Copyright 2012, the National Academy of Sciences of the United States of America.
