Abstract
Osteoarthritis (OA) is a leading cause of physical disability among aging populations, with no available drugs able to efficiently restore the balance between cartilage matrix synthesis and degradation. Also, OA has not been accurately classified into subpopulations, hindering the development toward personalized precision medicine.
In the present study, we identified a subpopulation of OA patients displaying high activation level of epidermal growth factor receptor (EGFR). With Col2a1-creERT2; Egfrf/f mice, it was found that the activation of EGFR, indicated by EGFR phosphorylation (pEGFR), led to the destruction of joints. Excitingly, EGFR inhibition prohibited cartilage matrix degeneration and promoted cartilage regeneration. The Food and Drug Administration (FDA)-approved drug gefitinib could efficiently inhibit EGFR functions in OA joints and restore cartilage structure and function in the mouse model as well as the clinical case report.
Overall, our findings suggested the concept of the EGFR activated OA subpopulation and illustrated the mechanism of EGFR signaling in regulating cartilage homeostasis. Gefitinib could be a promising disease-modifying drug for this OA subpopulation treatment.
Keywords: Osteoarthritis, Disease subpopulation, Epidermal growth factor receptor, Gefitinib
Highlights
-
•
There is a subpopulation of osteoarthritic patients with high activation level of EGFR (pEGFRhigh).
-
•
Gefitinib can simultaneously promote ECM synthesis and inhibit further degradation in pEGFRhigh osteoarthritic cartilage.
-
•
Intra-articular controlled-release of gefitinib could be a therapeutic strategy for pEGFRhigh OA treatment.
-
•
EGFR activation regulates OA development through inhibition of autophagy.
Osteoarthritis (OA) is one of the most prevalent musculoskeletal disorder worldwide, but most treatments in clinics are designed as a “one-size-fits-all-approach”. We found that not all OA patients are the same, and specifically in this case, there is a group of people with high activation level of EGFR, a functional protein in the cells of cartilage. The anti-cancer drug gefitinib was discovered to have multiple functions in protecting articular cartilage from destruction in EGFR activated OA. Our research therefore highlighted the OA subpopulation concept and suggested a novel OA therapeutic strategy.
1. Introduction
Osteoarthritis (OA) is one of the most prevalent musculoskeletal disorder affecting millions of people worldwide. In 2008, it was reported that 27 million Americans were afflicted by OA [1]; the number is projected to double by 2030 [2]. In China, 10% of males and 18% of females over the age of 60 were reported to have OA-related symptoms [3].
Precision medicine, or personalized medicine, is an innovative approach to tailoring disease prevention and treatment to the individual patient on the basis of a person's genes, lifestyle, and environment. However, the underlying concept of precision medicine has not yet been applied in OA as most treatments are designed as a “one-size-fits-all-approach” even with millions of patients suffering from the disease. This may be due to our limited understanding of the disease pathogenesis and mechanism; thus, more in-depth mechanistic studies need to be conducted.
Previous studies showed that the epidermal growth factor receptor (EGFR) signaling network played a role in cartilage development and OA [[4], [5], [6], [7], [8]]. Transforming growth factor-α (TGF-α), an activator of EGFR signaling, was reported to be involved in animal OA models, as well as in human [[9], [10], [11], [12]]. These studies indicated the involvement of EGFR in cartilage anabolic and catabolic activities.
Cartilage damage represents the most prominent pathological feature of OA that inevitably leads to joint dysfunction [13]. Disturbances of the balance between catabolic and anabolic activities is one of the main characteristics of OA cartilage; therefore, the restoration of the balance is the key factor for treating OA [14, 15]. However, currently there are no effective therapeutic drugs available for OA in clinics [16]. The non-steroidal anti-inflammatory drugs (NSAIDs) only have a palliative effect, mainly aimed at alleviating the pain instead of addressing the underlying cause of OA disease progression [17]. Recently, the disease-modifying OA drugs (DMOADs) in development were introduced [14, 15] but few of them affected both anabolic and catabolic aspects and showed convincing disease-modifying efficacy [14]. Therefore, it would be beneficial to find a drug that could simultaneously and synergistically regulate both cartilage matrix synthesis and degradation.
In the present study, we examined the activation level of EGFR in human patients, and utilized Col2a1-creERT2; Egfrf/f mice to investigate the effect of EGFR on OA development. Intra-articular delivery of EGFR inhibitor gefitinib, encapsulated in chitosan microsphere, was applied to treat OA in the mouse model. Moreover, the regulating mechanism of EGFR was investigated.
2. Methods and Materials
2.1. Collection and Preparation of Human Tissues
Cartilage specimens were from OA patients undergoing total knee replacement surgery or non-osteoarthritis trauma patients who were undergoing arthroscopic knee surgery. The harvested cartilage tissue samples were fixed, embedded in paraffin, sliced (7 μm) and mounted on positively charged slides for immunohistochemistry and safranin O staining.
2.2. OA Animal Model and Gefitinib Treatment
For Col2a1-creERT2; Egfrf/f and Egfrf/f male mice, tamoxifen (20 mg/ml) was administrated at the age of 2 months through intraperitoneal injection (5 μl/g) for 5 days. DMM surgery was performed 5 days after tamoxifen withdrawal, following the instructions as described previously [18]. The mice were sacrificed at 8 and 12 weeks after surgery and the joints were harvested. DMM surgery was also performed on normal 2-month old C57BL/6 male mice. For the sham surgery group, the knee joints were opened and then sutured without any treatment. For the systemic drug delivery experiment, DMM mice received either gefitinib (5, 25, 50 mg/kg) or vehicle control (normal saline, 0.9% NaCl) via oral gavage at 1-week post-surgery, once per day, for 8 weeks. For intra-articular treatment, chitosan microspheres with gefitinib (CM-Gefitinib) or CM in normal saline were injected into OA joints of mice, once every three days. The injections initiated at day 3 post-surgery and continued for 8 weeks until tissue samples were harvested for analysis. Each experimental group included a total of 6 mice.
2.3. Murine Tissue Fixation and Histology Processing
At the time of harvest, mice were euthanized and the surgically manipulated knee joints were dissected with the femur and tibia intact to maintain the structural integrity of the joint. Tissues were fixed and then decalcified in 10% (w/v) ethylene diamine tetraacetic acid (EDTA) solution. Subsequently, samples were embedded, sliced (7 μm) and numbered from 1 to 20. Sections numbered 5, 10 and 15 were stained with safranin O for OARSI scoring, while No. 6, 11, 16 sections were stained with Hematoxylin-Eosin (HE). Unstained sections were utilized for immunostaining.
2.4. OARSI and Synovial Inflammation Scoring of Murine Cartilage
Semi-quantitative histopathological scoring system established by the OA Research Society International (OARSI) was performed for grading mouse cartilage degeneration [19]. Summed OARSI scores were applied. Synovium was examined using the synovial inflammation grading system [20, 21]. Grading was performed by three blinded observers. The three grades for each section were averaged, and the data from each group of mice were collated.
2.5. Immunostaining
Paraffin sections for immunohistochemistry were treated with 0.4% pepsin (Sangon Biotech, Shanghai, China), 3% (v/v) hydrogen peroxide in methanol, 1% (w/v) BSA, primary antibodies and secondary antibodies subsequently. The DAB substrate system (ZSGB-bio, Beijing, China) was used for color development. Hematoxylin staining was utilized to reveal the cell nuclei. For quantitative analysis, 3 sections from different samples were selected for each group, and the positive/total cell ratio were calculated for each section.
Sections for immunofluorescence were incubated with 0.3% (v/v) Triton X-100, 1% (w/v) BSA, primary antibodies, corresponding secondary antibodies conjugated to Alexa Fluor 488 fluorescent dyes (Invitrogen) and DAPI subsequently. Images were viewed and captured under a confocal microscopy system (Olympus, BX61W1-FV1000, Japan and YOKOGAWA CV1000, Japan).
2.6. Primary Cultures of Mouse Chondrocytes
Mouse articular cartilage was obtained from the femoral condyles and tibial plateaus of postnatal day 5–6 C57BL/6 mice, and digested with 0.2% (w/v) collagenase overnight, as described previously [22]. Chondrocytes were maintained as a monolayer in DMEM/F12 supplemented with 10% (v/v) fetal bovine serum (FBS) at 37 °C, 5% CO2 environment. Cells between 0 and 3rd passage were utilized for experiments.
2.7. TGF-α and Gefitinib Treatment and In Vitro Gene Knockout
Chondrocytes were treated with 10 μM gefitinib or/and 10 ng/ml TGF-α in the culture medium. After 48 h, RNA and protein extraction were performed.
For in vitro gene knockout, isolated chondrocytes from Col2a1-creERT2; Egfrf/f mice were treated with 4-hydroxytamoxifen (1 μM) for 48 h before RNA and protein extraction. All in vitro experiments and assays were repeated 3 times.
2.8. qPCR Analysis
mRNA was extracted and reverse-transcripted followed by qPCR process. The relative level of expression of each target gene was calculated using the 2-ΔΔCt method. Each qPCR was performed on at least 3 different experimental samples with 3 technical replicates per sample. The representative results are displayed as target gene expression normalized to the reference gene Gapdh. Error bars represent one SD from the mean of technical replicates.
2.9. Western Blot Analysis
The proteins of human and mouse chondrocytes were directly extracted and the concentrations were determined using the BCA Protein Assay Kit (Pierce #23227). The extracted proteins were then separated on SDS-PAGE gels and transferred onto a polyvinylidene difluoride membrane. After blocked in 1% (w/v) BSA for 1 h at room temperature, the membrane was incubated with appropriate primary antibodies and horseradish peroxidase (HRP) conjugated secondary antibodies. The chemiluminescent signal was generated using western blot detection reagents (ECL, Beyotime Biotechnology, China and FDbio, Hangzhou, China) according to the manufacturer's protocol.
2.10. RNA-Sequencing and Data Analysis
Chondrocytes were treated with TGF-α or gefitinib as described previously and lysed by RNAiso Plus (TaKaRa). For each group, 2 duplicates were collected and the RNAs were extracted, sequenced, and analyzed by Shanghai Novel Bioinformatics Co, Ltd. (http://www.novelbio.com). EBseq algorithm was applied to filter the differentially expressed genes (DEGs). DEGs were then used for gene ontology (GO) and pathway analysis. Gene ontology enrichment analysis (GSEA) was performed using MATLAB based GSEA. The enrichment scores were normalized with Monte Carlo method [23]. RNA-seq raw data is available in Gene Expression Omnibus (GEO) database (GSE113271).
2.11. Fabrication of Chitosan Microspheres and Release Assay
Chitosan microspheres were prepared using the water-in-oil emulsion solvent diffusion method as previously described [24]. To evaluate the efficacy of chitosan microspheres (CM) delivery system, bovine serum albumin (BSA) was utilized as a substitute for gefitinib and was prepared by directly dropping chitosan solution into the oil phase under the same conditions. Three pieces of CM-BSA were mixed with 20 ml PBS and stored at 37 °C in a centrifuge tube to mimic body temperature. Solution (2 ml) was removed and resupplied at 0, 1, 3, 6, 12, 18, 24, 36, 48, 60, 72, 84, and 96 h. The cumulative release of BSA was tested with Micro BCA Protein Assay Kit (Thermo Scientific).
2.12. RNA Interference
In mouse chondrocytes, autophagy was suppressed by short hairpin RNAs (shRNA) targeting Atg5. All shRNAs were purchased from Invitrogen Inc. and two shRNAs with distinctive sequences were utilized.
2.13. Cartilage Explants
Cartilage explants were harvested from the weight-bearing area of the femoral articular surfaces of postnatal 24 h C57BL/6 mice knee joints. Explants were cultured for 24 h at 37 °C and 5% CO2 in DMEM supplemented with 10% (v/v) FBS at 37 °C and then treated with TGF-α, gefitinib or 3MA for one week.
2.14. Lentivirus Injection
ShAtg5 containing lentivirus was fabricated by Genepharma Inc. and the titer of the lentivirus was 1 × 109 U/ml. The shAtg5 2# was utilized. Intra-articular injections of LV-shAtg5 (10 μl per joint) were carried out at 10, 25, and 40 days post-DMM surgery to five C57/BL6 mice of the experimental group, and the control group mice received LV-scramble. Knee joints were collected 8 weeks post-surgery.
2.15. Statistics
All data for qPCR analysis and mouse OARSI scoring are presented as mean ± standard deviation (SD). Student's t-test, one-way ANOVA, Tukey's multiple comparisons test and non-parametric Mann-Whitney test were utilized accordingly. Values of P < 0.05 were considered to be statistically significant.
2.16. Study Approval
The patient's consent, as well as approval of the local ethics committee, were obtained prior to harvesting of human tissue samples. All animal experiments were performed with the approval of the Zhejiang University Ethics Committee (ZJU20160030).
More details are provided in supplementary material.
3. Results
3.1. EGFR Signaling Was Involved in Human pEGFRhigh OA Subpopulation
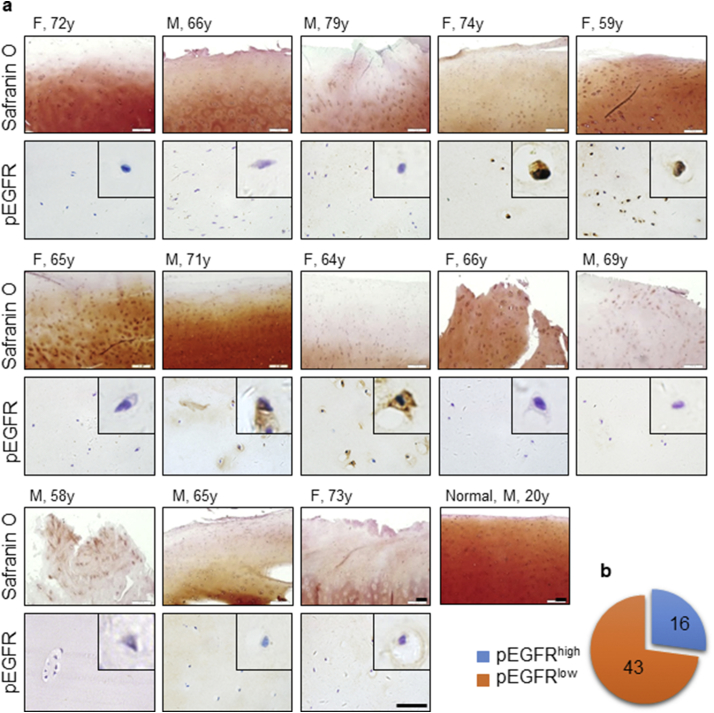
We first investigated the involvement of EGFR in human OA samples. A total of 59 human knee OA cartilage samples (Table 1) were collected. The patients were previously evaluated by Kellgren and Lawrence Grading System (K&L) and graded between 3 and 4. All OA samples from different individuals showed a reduction in proteoglycan level and defective cartilage surface indicated by Safranin O staining when compared to normal cartilage (Fig. 1a), confirming their osteoarthritic properties. EGFR activation state was then determined by immunostaining of the phosphorylated EGFR. To our surprise, only 27% of the total collected OA samples had a strong positive staining of pEGFR (pEGFRhigh) (Fig. 1a, b and S1a), with no total EGFR level alteration (Fig. S1b), indicating the heterogeneity of OA population; and the proportion was relatively higher in females (Table 1). Meanwhile, no positive staining was detected in normal cartilages from 7 relatively young individuals (Fig. S1c). Therefore, we concluded that the EGFR-activated OA could be one of the various subpopulations of OA.
Table 1.
Gender, age and pEGFRhigh proportion of the OA patients.
| Qty | Age (years) |
pEGFRhigh | Positive% | |||
|---|---|---|---|---|---|---|
| Min | Max | Average | ||||
| Male | 19 | 58 | 79 | 70.7 | 2 | 10.35% |
| Female | 40 | 51 | 82 | 68.3 | 14 | 35.00% |
| Total | 59 | 69.1 | 16 | 27.11% | ||
Fig. 1.
EGFR was activated in a subpopulation of human OA patients. (a) Safranin O staining and Immunostaining of the human OA samples (n = 59). F, female; M, male. (b) The proportion of the pEGFRhigh subpopulation in collected samples.
3.2. EGFR Activation Promoted OA Progression by Disrupting Cartilage Homeostasis
In order to investigate the function of EGFR in OA pathogenesis, we employed the inducible conditional knockout mouse (Col2a1-creERT2; Egfrf/f) and isolated chondrocytes from the Col2a1-creERT2; Egfrf/f mice and the Egfrf/f siblings. The in vitro knockout was induced by 4OH-tamoxifen; the efficiency was approximately 76% confirmed by qPCR and western blot (Fig. S2a). Then TGF-α stimulation was utilized to activate EGFR. For wild-type (WT) cells, results illustrated that 48 h after TGF-α stimulation, genes encoding cartilage matrix type II collagen (Col2a1) and aggrecan (Acan) were downregulated while the matrix-degrading enzyme matrix metalloproteinase 13 (Mmp13) was upregulated (Fig. 2a), indicating that EGFR activation interrupted the balance between matrix biosynthesis and degradation. However, after Egfr knockout, the effects of TGF-α were suppressed as demonstrated by the unaltered Acan, Col2a1 and Mmp13 mRNAs, as well as MMP13 and type II collagen proteins (COL2) (Fig. 2b). These results suggested that EGFR activation was responsible for the loss of chondrocyte homeostasis in vitro.
Fig. 2.
EGFR activation promoted OA progression by disrupting cartilage homeostasis. (a and b) Cartilage ECM expression level detection with or without TGF-α stimulation by quantitative real-time polymerase chain reaction (qPCR) and western blot. WT, wild-type; KO, knockout; ***P < 0.001; ns = not significant. (c) Safranin O and immunostaining of the mouse knee joints 8 weeks (left panel) and 12 weeks (right panel) post-surgery (n = 6). WT, wild-type (Egfrf/f); CKO, conditional knockout (Col2a1-creERT2; Egfrf/f). Scale bar, 100 μm. (d) Quantitative analysis of the mouse knee joints 8 weeks (left panel) and 12 weeks (right panel) post-surgery. *P < 0.05; **P < 0.01.
Destabilization of the medial meniscus (DMM) is widely used to induce OA in mice [18, [25], [26], [27]]. We performed DMM surgery on the Col2a1-creERT2; Egfrf/f (CKO) mice as well as their Egfrf/f (WT) siblings after knockout induction by tamoxifen. Immunostaining of EGFR on the articular cartilage confirmed the knockout efficacy of the inducible conditional knockout system in vivo (Fig. S2b). Positive staining of the phosphorylated EGFR (pEGFR) in the articular cartilage of the DMM mice 8 weeks after surgery demonstrated that EGFR was activated in this OA model (Fig. S3). Safranin O staining of the DMM joints demonstrated thicker and smoother joint surfaces in the Egfr conditional knockout group with significantly lower OARSI scores at 8 weeks and 12 weeks post-surgery (Fig. 2c, d). Immunostaining also displayed well-maintained extracellular matrix (ECM) proteins including type II collagen and aggrecan and a downregulation of MMP13 in Col2a1-creERT2; Egfrf/f mice when compared with WT siblings at both time points (Fig. 2c, d). Meanwhile, there was no significant alteration in the articular cartilage after 8 weeks of Egfr knockout in the sham group (Fig. S4). These data indicated that the inhibition of EGFR activation in Col2a1-creERT2; Egfrf/f mice prevented OA progression and restored cartilage homeostasis by elevating ECM content and decreasing MMP13 expression; Egfr knockout alone in adult mice didn't impair the knee articular cartilage.
3.3. EGFR Inhibition by Gefitinib Efficiently Promoted Cartilage Homeostasis Maintenance
Gefitinib is an FDA-approved drug for EGFR inhibition. As we demonstrated that EGFR inactivation in Col2a1-creERT2; Egfrf/f mice protected the mice from OA progression and restored cartilage homeostasis, we next questioned whether gefitinib could also be used for OA treatment. Western blot data revealed that TGF-α activated EGFR, but gefitinib was able to inhibit the activity of EGFR as shown by the decreased pEGFR level (Fig. 3a). Consistently, TGF-α treatment inhibited COL2 and upregulated MMP13 expression; however, these effects could also be reversed by gefitinib (Fig. 3a and S5). In addition, qPCR analysis demonstrated the downregulation of Col2a1 and Acan after TGF-α stimulation (Fig. 3b), indicating that the loss of ECM content was not only caused by degradation but also through the suppression of ECM biosynthesis. Gefitinib was able to inhibit ECM degradation and promote its synthesis simultaneously in the presence of TGF-α, while gefitinib alone didn't alter Col2a1, Acan, and Mmp13 gene expression (Fig. 3b).
Fig. 3.
EGFR inhibition of gefitinib efficiently promoted cartilage homeostasis maintenance. (a) Detection of the effect of TGF-α and gefitinib on mouse chondrocytes by western blot. T, TGF-α; G, gefitinib. (b) Detection of the effect of TGF-α and gefitinib on mouse chondrocyte ECM and degradation enzyme expression level by qPCR. **P < 0.01; ***P < 0.001. (c) Gene Ontology (GO) analysis of the genes which were downregulated after TGF-α stimulation and reversed by gefitinib. (d) Gene Set Enrichment Analysis (GSEA) of the cartilage anabolic and catabolic functions. (e) QPCR of the cartilage-related genes for RNA-seq validation. *P < 0.05; **P < 0.01; ***P < 0.001.
To further confirm this on the transcriptomic scale, RNA sequencing was performed. Principle component analysis (PCA) and differential expression gene analysis showed a similar expression profile in the control and TGF-α + gefitinib (T + G) group, while the TGF-α group showed a distinct gene expression profile (Fig. S6), signifying that gefitinib reversed the alterations induced by TGF-α stimulation. Gene ontology (GO) analysis showed several GO terms related to cartilage ECM synthesis in the top-related lists such as extracellular matrix and its organization, cartilage development, collagen fibril organization, etc. (Fig. 3c and S7a). Gene set enrichment analysis (GSEA) showed that genes related to collagen biosynthetic process, collagen fibril organization, collagen network, and positive regulation of extracellular matrix organization were down-regulated with TGF-α stimulation and reversed with T + G treatment. Similarly, collagen catabolic process-related genes were upregulated with TGF-α stimulation, and the effects were also reversed with T+G treatment (Fig. 3d and S7b). The sequencing data was further validated by qPCR of some highly related genes including Bmp4, Col1a1, Col9a1, Col11a1, Col11a2, Comp, Fgfr3, Grem1, Lect1, Sox6, Sparc and Tgfb2 (Fig. 3e). These data supported that EGFR inhibition could promote cartilage homeostasis maintenance in OA, and this goal can be achieved using gefitinib.
3.4. Intra-Articular Controlled-Release of Gefitinib Ameliorated OA Progression in the Mouse Model
Gefitinib has been orally administered in clinical practice for decades, and our histological results with this method showed remarkable potential in ameliorating OA progression in the DMM mouse model (Fig. 4a). However, severe side effects were also observed including hair loss, cachexia, and even death when administrated in high dose (50 mg/kg); thus, chitosan microspheres (CM) were utilized as a tool for local drug delivery and controlled-release of gefitinib. The CM and gefitinib-loaded CM (CM-gefitinib) microspheres displayed good spherical structures and surface uniformity while no significant differences or changes were observed in the drug-loaded CMs under scanning electronic microscope (SEM) (Fig. 4b). Fourier Transform Infrared Spectroscopy (FTIR) confirmed that gefitinib was enclosed in chitosan (Fig. 4c). The drug release profile from CMs was tested; results showed >90% cumulative release of the CM-enclosed BSA in 72 h (Fig. 4d).
Fig. 4.
Intra-articular controlled-release of gefitinib ameliorated OA progression in the mouse model. (a) Safranin O staining and the OARSI scores of the mouse joints with or without systemic administration of gefitinib (n = 6). Scale bar, 100 μm; **P < 0.01. (b) Scanning electron micrographs of unloaded chitosan microspheres (CM) and gefitinib-loaded chitosan microspheres (CM-gefitinib). (c) Fourier Transform Infrared Spectroscopy (FTIR) for chitosan microspheres. (d) The controlled release profile of chitosan microspheres. (e) Immunostaining of the pEGFR in control and OA joints with CM-gefitinib (n = 6). NS, normal saline; Scale bar, 100 μm. (f) Safranin O staining of the pEGFR in control and OA joints with CM-gefitinib (n = 6). Scale bar, 100 μm. (g) Quantitative analysis of Safranin O and immunostaining. *P < 0.05; **P < 0.01.
Then the drug delivery system was tested on DMM mouse model. An intra-articular injection of CM-gefitinib, CM, or normal saline (NS) was performed starting from day 3 post-surgery. The drug was administrated once every 3 days for 8 weeks before the mice were sacrificed. Staining results showed that pEGFR was expressed in fewer chondrocytes (Fig. 4e, g) and the severity of these osteoarthritic-like changes was reduced in the CM-gefitinib-treated mice when compared with the NS- or CM-treated OA mice (Fig. 4f, g). Reduced synovial inflammation in response to CM-gefitinib treatment was observed as well (Fig. S8), showing good biocompatibility of the material. Consistently, gefitinib was also shown to promote cartilage matrix deposition during rats' osteochondral defect repair when applied together with gelatin methacrylate-silk fibroin (GelMA-SF) scaffold (Fig. S9). These results indicated that the local controlled-release of gefitinib in joints could facilitate the maintenance of cartilage homeostasis through the inhibition of EGFR signaling.
3.5. EGFR Activation Promoted OA Progression through Autophagy Inhibition
We next investigated how EGFR inhibition promotes cartilage homeostasis. Histone acetylation transforms the condensed chromatin structure into a more relaxed form that is associated with greater levels of gene transcription. Therefore, we performed chromatin immunoprecipitation followed by sequencing (ChIP-Seq) for the acetylated H3K27 (H3K27Ac) in TGF-α and gefitinib treated mouse chondrocytes. Differentially modified genes (highly acetylated in control and T + G groups but not in TGF-α group) were clustered based on gene ontology; GO data implicated that autophagy-related genes were enriched (Fig. S10a), consistent with the GSEA result of our RNA-seq data (Fig. S10b). Hence, we examined the autophagy-related proteins, LC3 and Beclin1, [28] in vivo in the DMM mouse model. As expected, LC3 and Beclin1 were highly expressed in the normal cartilage while significantly lower levels were detected in OA cartilage. Deletion of EGFR in Col2a1-creERT2; Egfrf/f cartilage could recover the expression level of LC3 and Beclin1 (Fig. 5a). In vitro validation also illustrated that LC3-II, the active form of LC3 and one of the main components of the autophagosome, was increased and more punctate stains of LC3 (showing an autophagy-active state) appeared in the gefitinib-treated and T + G groups when compared to the TGF-α-induced EGFR-activated group, thus indicating that the inhibition of EGFR could remarkably increase autophagy activity (Fig. 5b, c). Next, we employed short hairpin RNAs (shRNAs) to knockdown the autophagy-related gene 5 (Atg5) which is necessary for LC3-II formation. Results revealed that the inhibition of Atg5 aborted the protective function of gefitinib as indicated by the suppressed LC3-II and COL2 levels and increased MMP13 level when compared with the scrambled group (Fig. 5d). Consistently, mRNA expression of Col2a1 and Mmp13 were also affected by Atg5 shRNAs, halting the protective function of gefitinib (Fig. 5e).
Fig. 5.
EGFR activation promoted OA progression through inhibiting autophagy. (a) Immunostaining of the autophagy-related proteins in mouse sham operated, and OA cartilage (n = 6). **P < 0.01; ***P < 0.001; Scale bar, 100 μm. (b) Detection of LC3 with TGF-α and gefitinib stimulation by western blot. (c) Immunostaining of LC3 on TGF-α and gefitinib-treated cells. Scale bar, 25 μm. (d) ATG5 knockdown efficiency and the impact on LC3, COL2 and MMP13 detected by western blot. (e) Col2a1 and Mmp13 gene expression level detection after ATG5 knockdown by qPCR. **P < 0.01; ***P < 0.001. (f) Immunostaining and Safranin O staining of the cartilage explant treated with TGF-α, gefitinib or 3MA. Yellow arrows indicate ECM loss. Yellow scale bar, 50 μm; black scale bar, 100 μm. (g) Immunostaining of the autophagy-related proteins Beclin1 and LC3 in control and OA joints with CM-gefitinib (n = 6). Scale bar, 100 μm. (h) Safranin O staining and OARSI scores of mouse knee joints treated with DMM surgery (OA), CM-Gefitinib and shAtg5 lentivirus (LV-shAtg5) (n = 5). Scale bar, 100 μm; *P < 0.05.
To mimic the in vivo environment, we utilized a cartilage explant model to further confirm the protective effects of gefitinib on cartilage in 3D culture. Results revealed a decrease in safranin O staining, COL2 and LC3 levels and an increase in MMP13 level in TGF-α-treated cartilage explants but all reversed when treated with gefitinib. Nevertheless, the protective effect of gefitinib was revoked by 3-Methyladenine (3MA), an autophagy inhibitor (Fig. 5f).
We then checked autophagy-related proteins in CM-gefitinib treated OA mice. Immunostaining analysis showed that Beclin1 and LC3 expressions were enhanced in CM-gefitinib-treated mice when compared with OA-CM-treated mice (Fig. 5g). However, the injection of shAtg5-containing lentivirus (LV-shAtg5) abolished the protective effects of gefitinib in OA mice (Fig. 5h and S11). These observations suggested that the activation of autophagy by gefitinib impeded OA pathological changes, while the inhibition of autophagy diminished the positive therapeutic effects of gefitinib.
Moreover, our data also illustrated that expression levels of autophagy-related factors LC3 and Beclin1 in pEGFRhigh OA samples were significantly lower when compared to normal and pEGFRlow samples (Fig. S12), implying that the pathogenesis of the pEGFRhigh OA subpopulation may have a similar mechanism with the animal model.
4. Discussion
In the present study, we discovered several major original findings: 1) there is a pEGFRhigh subpopulation of around 27% in OA patients, 2) EGFR activation is involved in OA progression through the regulation of cartilage matrix synthesis and degradation, and 3) suppression of EGFR by the FDA-approved drug gefitinib re-activated chondrocyte autophagy and restored cartilage homeostasis by simultaneously promoting cartilage matrix synthesis and prohibiting its degradation. These findings are of great value for the future development of personalized therapeutic application to restore cartilage homeostasis for the subpopulation of OA patients with high EGFR activation.
Because OA is the leading type of arthritis affecting millions of patients worldwide, it is logical to speculate that OA patients are likely heterogeneous. In this study, we found that approximately a quarter (27%) of human samples were positively stained with pEGFR, similar to the highly EGFR-activated mouse DMM joints. We also demonstrated that EGFR suppression in mouse samples with conditional gene knockout technology can prohibit OA development progression. Although the ratio of EGFR high activation was different between human and the mouse DMM model, there is no doubt to conclude that EGFR is involved in the OA development. Furthermore, since not all human samples were pEGFRhigh, and our in vivo study showed no impairment on the articular cartilage after Egfr knockout in adult mice while other studies reported that consistent suppression of EGFR activity from the embryonic stage, rather than just before OA occurrence, accelerated OA development, [29, 30] it is possible that EGFR signaling exerts differential functions at a particular stage or type. Therefore, the 27% of EGFR activation in human OA patients could either be a sub-stage or subtype of OA, similar to the previously reported EGFR lung and gastric cancer subtype [[31], [32], [33], [34]]. Consistently, a case report informed that a 72-year-old woman with non-small-cell lung carcinoma (NSCLC) showed a remarkable improvement in her arthritis symptoms since the first dose of gefitinib [35]. The proteomic study also showed that EGFR signaling-related proteins were enriched in OA patients [36]. In any case, there is a subpopulation with high activation level of EGFR, and our findings warrant the necessity of investigating the EGFR-targeting treatment strategies for pEGFRhigh OA patients, which is crucial for the development of precision medicine in OA disease.
Cartilage homeostasis is maintained by the balance between matrix synthesis and degradation. In this study, in vitro and in vivo experiments showed an upregulation of cartilage ECM component biosynthesis and a downregulation of the matrix degradation enzyme MMP13 after EGFR suppression; thus, indicating the two important roles of EGFR in cartilage homeostasis maintenance. Consistently, EGFR was reported responsible for chondrocyte maturation and calcification in development, [37] which is also one of the pathophysiological ossification processes in OA. OA-like characteristics could be seen in cartilage-specific Mig6 (an intrinsic EGFR inhibitor) knockout mice, especially in the knee joints, where EGFR signaling was highly activated [6]. These findings suggested that EGFR is a promising therapeutic target for OA. Furthermore, the present study also revealed that EGFR may regulate cartilage homeostasis through autophagy. While autophagy was previously demonstrated to be essential in cartilage homeostasis maintenance, [38] OA progression, [28, 39, 40] and several reports illustrated that inhibition of EGFR up-regulated autophagy in tumor cells, [41, 42] our results, together with previous research findings, connected the missing linkage between EGFR, autophagy, and downstream effectors type II collagen and MMP13 in OA cartilage. Nevertheless, more research is needed.
Based on the findings from EGFR gene knockout OA mice, the EGFR inhibitor gefitinib was used to restore cartilage homeostasis and treat pEGFRhigh OA through systemic or local drug delivery. Our results showed that gefitinib possessed a great benefit in both anabolic and catabolic aspects. Since gefitinib is an FDA-approved drug, it can be more feasibly translated into clinical application. However, the anti-tumor drug was shown to have severe side effects after systemic delivery; it was also shown in our study and another research that high dose of gefitinib by systemic delivery accelerated OA progression and weight loss [30]. Therefore, chitosan microspheres were employed in this study as a local drug delivery system because of its biocompatible, non-toxic, and cost-effective characteristics [24, 43]. As expected, intra-articular delivery of low dose gefitinib encapsulated in chitosan microspheres exhibited significant OA therapeutic effect with no apparent systemic side effects or local synovitis occurrence, which diminished the concern that gefitinib might exacerbate synovitis formation in degenerating joints [44]. The current controlled-release system guarantees an approximately 3-day sustained-release of gefitinib, demonstrating a very promising local delivery strategy for OA treatment; however, further optimization of the system is needed.
In summary, our findings raised a concept of heterogeneous OA subpopulation, particularly, in this case, the pEGFRhigh OA subpopulation. The critical role of EGFR in OA development and therapeutic applications made EGFR a promising target of pharmacotherapy. The FDA-approved drug gefitinib can readily be translated into disease-modifying clinical therapy for OA. Thus, the findings provide new insights into OA pathophysiology and future precise therapeutic strategy for OA treatment.
Acknowledgments
Acknowledgements
The authors thank Varitsara Bunpetch for the manuscript preparation, Dr. Chen Di for kindly providing Col2a1-CreERT2 mice, Dr. David Threadgill for providing Egfrf/f mice, and Shanghai Novel Bioinformatics Co. for RNA-Seq analysis.
Funding Sources
This work was supported by the National Key R&D Program of China (2017YFA0104902, 2016YFB0700804), Natural Science Foundation of China (81630065, GZ1094, 81472115, 81672162) and Science and Technology Department of Zhejiang Province (2013TD11).
Conflicts of Interest
We declare that we have no conflicts of interest.
Author Contributions
H·S, Y·W and H·O designed the research; H·S, Y·W, D.Y, XA.Z, H·W, XL.Z and T.Q performed the molecular experiments; H·S, Y·W, D.Y, P·C, Z.P and X.L performed the animal experiments; C.A, Y·C and C·Y analyzed the data; H·S, Y·W, W.Z, S.Z and H·O wrote the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.06.002.
Appendix A. Supplementary Data
Supplementary material
References
- 1.Lawrence R.C., Felson D.T., Helmick C.G., Arnold L.M., Choi H., Deyo R.A. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helmick C.G., Felson D.T., Lawrence R.C., Gabriel S., Hirsch R., Kwoh C.K. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 3.Tang X., Wang S., Zhan S., Niu J., Tao K., Zhang Y. The prevalence of symptomatic knee osteoarthritis in China: results from the China health and retirement longitudinal study. Arthritis Rheumatol. 2016;68:648–653. doi: 10.1002/art.39465. [DOI] [PubMed] [Google Scholar]
- 4.Shepard J.B., Jeong J.W., Maihle N.J., O'Brien S., Dealy C.N. Transient anabolic effects accompany epidermal growth factor receptor signal activation in articular cartilage in vivo. Arthritis Res Ther. 2013;15:R60. doi: 10.1186/ar4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin S.Y., Pozzi A., Boyd S.K., Clark A.L. Integrin alpha1beta1 protects against signs of post-traumatic osteoarthritis in the female murine knee partially via regulation of epidermal growth factor receptor signalling. Osteoarthritis Cartilage. 2016;24:1795–1806. doi: 10.1016/j.joca.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Staal B., Williams B.O., Beier F., Vande W.G., Zhang Y.W. Cartilage-specific deletion of Mig-6 results in osteoarthritis-like disorder with excessive articular chondrocyte proliferation. Proc Natl Acad Sci U S A. 2014;111:2590–2595. doi: 10.1073/pnas.1400744111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X., Siclari V.A., Lan S., Zhu J., Koyama E., Dupuis H.L. The critical role of the epidermal growth factor receptor in endochondral ossification. J Bone Miner Res. 2011;26:2622–2633. doi: 10.1002/jbmr.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y.W., Su Y., Lanning N., Swiatek P.J., Bronson R.T., Sigler R. Targeted disruption of Mig-6 in the mouse genome leads to early onset degenerative joint disease. Proc Natl Acad Sci U S A. 2005;102:11740–11745. doi: 10.1073/pnas.0505171102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appleton C.T., Usmani S.E., Bernier S.M., Aigner T., Beier F. Transforming growth factor alpha suppression of articular chondrocyte phenotype and Sox9 expression in a rat model of osteoarthritis. Arthritis Rheum. 2007;56:3693–3705. doi: 10.1002/art.22968. [DOI] [PubMed] [Google Scholar]
- 10.Appleton C.T., Usmani S.E., Mort J.S., Beier F. Rho/ROCK and MEK/ERK activation by transforming growth factor-alpha induces articular cartilage degradation. Lab Invest. 2010;90:20–30. doi: 10.1038/labinvest.2009.111. [DOI] [PubMed] [Google Scholar]
- 11.Appleton C.T., Usmani S.E., Pest M.A., Pitelka V., Mort J.S., Beier F. Reduction in disease progression by inhibition of transforming growth factor alpha-CCL2 signaling in experimental posttraumatic osteoarthritis. Arthritis Rheumatol. 2015;67:2691–2701. doi: 10.1002/art.39255. [DOI] [PubMed] [Google Scholar]
- 12.Usmani S.E., Ulici V., Pest M.A., Hill T.L., Welch I.D., Beier F. Context-specific protection of TGFalpha null mice from osteoarthritis. Sci Rep. 2016;6 doi: 10.1038/srep30434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Berg W.B. Osteoarthritis year 2010 in review: pathomechanisms. Osteoarthritis Cartilage. 2011;19:338–341. doi: 10.1016/j.joca.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Hunter D.J. Pharmacologic therapy for osteoarthritis—the era of disease modification. Nat Rev Rheumatol. 2011;7:13–22. doi: 10.1038/nrrheum.2010.178. [DOI] [PubMed] [Google Scholar]
- 15.Jotanovic Z., Mihelic R., Sestan B., Dembic Z. Emerging pathways and promising agents with possible disease modifying effect in osteoarthritis treatment. Curr Drug Targets. 2014;15:635–661. doi: 10.2174/1389450115666140306153115. [DOI] [PubMed] [Google Scholar]
- 16.Roubille C., Pelletier J.P., Martel-Pelletier J. New and emerging treatments for osteoarthritis management: will the dream come true with personalized medicine? Expert Opin Pharmacother. 2013;14:2059–2077. doi: 10.1517/14656566.2013.825606. [DOI] [PubMed] [Google Scholar]
- 17.Hochberg M.C., Altman R.D., April K.T., Benkhalti M., Guyatt G., McGowan J. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64:465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 18.Glasson S.S., Blanchet T.J., Morris E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Glasson S.S., Chambers M.G., Van Den Berg W.B., Little C.B. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl. 3):S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Carames B., Olmer M., Kiosses W.B., Lotz M.K. The relationship of autophagy defects to cartilage damage during joint aging in a mouse model. Arthritis Rheumatol. 2015;67:1568–1576. doi: 10.1002/art.39073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krenn V., Morawietz L., Haupl T., Neidel J., Petersen I., Konig A. Grading of chronic synovitis—a histopathological grading system for molecular and diagnostic pathology. Pathol Res Pract. 2002;198:317–325. doi: 10.1078/0344-0338-5710261. [DOI] [PubMed] [Google Scholar]
- 22.Gosset M., Berenbaum F., Thirion S., Jacques C. Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 2008;3:1253–1260. doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
- 23.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu S., Lu P., Liu H., Chen P., Wu Y., Wang Y. Inhibition of Rac1 activity by controlled release of NSC23766 from chitosan microspheres effectively ameliorates osteoarthritis development in vivo. Ann Rheum Dis. 2015;74:285–293. doi: 10.1136/annrheumdis-2013-203901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai J., Yu D., Wang Y., Chen Y., Sun H., Zhang X. Kdm6b regulates cartilage development and homeostasis through anabolic metabolism. Ann Rheum Dis. 2017;76:1295–1303. doi: 10.1136/annrheumdis-2016-210407. [DOI] [PubMed] [Google Scholar]
- 26.Kim J.H., Jeon J., Shin M., Won Y., Lee M., Kwak J.S. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell. 2014;156:730–743. doi: 10.1016/j.cell.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Wu C.L., Jain D., McNeill J.N., Little D., Anderson J.A., Huebner J.L. Dietary fatty acid content regulates wound repair and the pathogenesis of osteoarthritis following joint injury. Ann Rheum Dis. 2015;74:2076–2083. doi: 10.1136/annrheumdis-2014-205601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carames B., Taniguchi N., Otsuki S., Blanco F.J., Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia H., Ma X., Tong W., Doyran B., Sun Z., Wang L. EGFR signaling is critical for maintaining the superficial layer of articular cartilage and preventing osteoarthritis initiation. Proc Natl Acad Sci U S A. 2016;113:14360–14365. doi: 10.1073/pnas.1608938113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X., Zhu J., Liu F., Li Y., Chandra A., Levin L.S. Reduced EGFR signaling enhances cartilage destruction in a mouse osteoarthritis model. Bone Res. 2014;2 doi: 10.1038/boneres.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong L., Han Y., Brain L. The role of epidermal growth factor receptor in prognosis and treatment of gastric cancer. Expert Rev Gastroenterol Hepatol. 2014;8:111–117. doi: 10.1586/17474124.2014.844648. [DOI] [PubMed] [Google Scholar]
- 32.Mahmood M.Q., Shukla S.D., Dua K., Shastri M.D. The role of epidermal growth factor receptor in the management of gastrointestinal carcinomas: present status and future perspectives. Curr Pharm Des. 2017;23:2314–2320. doi: 10.2174/1381612823666170124115159. [DOI] [PubMed] [Google Scholar]
- 33.Siegelin M.D., Borczuk A.C. Epidermal growth factor receptor mutations in lung adenocarcinoma. Lab Invest. 2014;94:129–137. doi: 10.1038/labinvest.2013.147. [DOI] [PubMed] [Google Scholar]
- 34.Tang E.R., Schreiner A.M., Pua B.B. Advances in lung adenocarcinoma classification: a summary of the new international multidisciplinary classification system (IASLC/ATS/ERS) J Thorac Dis. 2014;6:S489–S501. doi: 10.3978/j.issn.2072-1439.2014.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moryl N., Obbens E.A., Ozigbo O.H., Kris M.G. Analgesic effect of gefitinib in the treatment of non-small cell lung cancer. J Support Oncol. 2006;4:111. [PubMed] [Google Scholar]
- 36.Tsolis K.C., Bei E.S., Papathanasiou I., Kostopoulou F., Gkretsi V., Kalantzaki K. Comparative proteomic analysis of hypertrophic chondrocytes in osteoarthritis. Clin Proteomics. 2015;12:12. doi: 10.1186/s12014-015-9085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X., Zhu J., Li Y., Lin T., Siclari V.A., Chandra A. Epidermal growth factor receptor (EGFR) signaling regulates epiphyseal cartilage development through beta-catenin-dependent and -independent pathways. J Biol Chem. 2013;288:32229–32240. doi: 10.1074/jbc.M113.463554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lotz M.K., Carames B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat Rev Rheumatol. 2011;7:579–587. doi: 10.1038/nrrheum.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carames B., Taniguchi N., Seino D., Blanco F.J., D'Lima D., Lotz M. Mechanical injury suppresses autophagy regulators and pharmacologic activation of autophagy results in chondroprotection. Arthritis Rheum. 2012;64:1182–1192. doi: 10.1002/art.33444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasaki H., Takayama K., Matsushita T., Ishida K., Kubo S., Matsumoto T. Autophagy modulates osteoarthritis-related gene expression in human chondrocytes. Arthritis Rheum. 2012;64:1920–1928. doi: 10.1002/art.34323. [DOI] [PubMed] [Google Scholar]
- 41.Tan X., Thapa N., Sun Y., Anderson R.A. A kinase-independent role for EGF receptor in autophagy initiation. Cell. 2015;160:145–160. doi: 10.1016/j.cell.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei Y., Zou Z., Becker N., Anderson M., Sumpter R., Xiao G. EGFR-mediated Beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell. 2013;154:1269–1284. doi: 10.1016/j.cell.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alpar H.O., Somavarapu S., Atuah K.N., Bramwell V.W. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv Drug Deliv Rev. 2005;57:411–430. doi: 10.1016/j.addr.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Goldring M.B., Goldring S.R. Osteoarthritis. J Cell Physiol. 2007;213:626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material