Abstract
We report on a case of disseminated CNS hemangioblastoma, also referred to as hemangioblastomatosis, involving the supratentorial compartment and the entire spine. The patient presented with new onset headache, gait difficulties and memory deficits many years following resection of a hemangioblastoma from the cerebellum. The patient's family history was negative for von Hippel–Lindau (VHL) disease, and his personal history was negative for any additional VHL-defining lesions. Imaging revealed extensive dural caking and nodularity both supratentorially and in the spine, along with scattered parenchymal tumors showing a more typical appearance for hemangioblastoma. Biopsy of the dural thickening revealed histologic features compatible with hemangioblastoma. Genetic testing for VHL was eventually completed, and no evidence of a germline VHL mutation was detected.
KEYWORDS : disseminated hemangioblastoma, hemangioblastoma, hemangioblastomatosis, VHL, von Hippel–Lindau
Practice points.
Widespread CNS dissemination of hemangioblastoma (hemangioblastomatosis) is a rare occurrence.
Hemangioblastomatosis is almost always found in the context of von Hippel–Lindau (VHL) disease.
Hemangioblastomatosis outside of VHL is possible but exceptionally rare. The finding of multiple or disseminated hemangioblastomas should prompt a search for indicators of VHL in the past medical or family histories, on imaging studies, and with genetic screening.
Hemangioblastoma is a relatively common primary CNS neoplasm, most often encountered in adults. The majority of hemangioblastomas are sporadic and nonfamilial, and these cases typically present with one or very few lesions in the cerebellum [1]. Less commonly, hemangioblastoma is encountered in the context of von Hippel–Lindau (VHL), in which case multiple tumors are often found in the cerebellum, brainstem, spinal cord and rarely the cerebral hemispheres. Hemangioblastoma associated with VHL presents at an earlier age than the sporadic variety [2]. Consequently, the identification of a hemangioblastoma in a pediatric patient or young adult should prompt screening for VHL.
Hemangioblastoma is a vascular lesion with well-circumscribed margins and little or no tendency to infiltrate the surrounding parenchyma [3]. The tumor is composed of two predominant cell types: stromal cells which are typically large and vacuolated, and vascular cells in the form of numerous small vessels. It is unclear which cell type – stromal, vascular, or both – represents the driving neoplastic cell. Hemangioblastoma is considered a benign tumor (WHO grade I) and in most cases behaves indolently. No histologic difference exists between sporadic and VHL-associated tumors, nor between disseminating and nondisseminating tumors. Hemangioblastoma is amenable to surgical cure, and prognosis is good for isolated lesions outside of VHL. Disseminated hemangioblastoma is a rare complication, much more commonly seen in VHL patients, and carries a poorer prognosis [1,4–7].
On MRI, hemangioblastoma presents as either a solid enhancing nodule or a cyst with an enhancing mural nodule [8–12]. The vast majority of lesions are found in the cerebellum. The lesion is vascular, and as a result, multiple prominent vessels can often be seen on imaging within or around the lesion. Microscopic or macroscopic hemorrhage may also be present. In the spine, a small, solidly enhancing nodule is most typical, with an occasional associated cyst or syrinx. Lesions are most commonly found along the dorsal surface of the cord, in a peripheral or ‘subpial’ location. Enlarged serpiginous vessels are usually quite conspicuous coursing along the cord surface above and below the tumor, a finding which helps to narrow the radiologic differential diagnosis. Occasionally, intradural-extramedullary lesions are found, usually in association with spinal nerve roots. Regardless of lesion location, extensive surrounding edema, out of proportion to the size of the tumor, is a hallmark of hemangioblastoma.
Case report
A 74-year-old male presented to our neuro-oncology clinic seeking advice on further management for multiple CNS hemangioblastomas. The patient stated that he was taken to surgery for removal of a cerebellar hemangioblastoma 12 years prior to the current presentation. The patient denied any personal or family history of VHL disease, and his prior workup was negative for any other VHL-defining lesions (e.g., renal neoplasm, retinal angioma, pheochromocytoma and abdominal organ cysts). Recently, the patient had been experiencing new headache, gait difficulties and dementia-like memory issues, which led to MR scans of the brain and spine. Imaging showed evidence of a prior occipital craniotomy as well as several lesions characteristic of parenchymal hemangioblastoma in the cerebellum (Figure 1). However, an unusual pattern of dural nodularity and caking was also seen along the anterior interhemispheric falx with significant edema in the adjacent frontal lobes (Figure 2). In much of the spine, a similar pattern of dural caking was also present, along with scattered more typical subpial cord hemangioblastomas, accompanied by the characteristic prominent vasculature (Figure 3). As the etiology for the dural abnormality was not clear, the patient was taken again to surgery for biopsy of the thick tissue along the interhemispheric falx. The histomorphologic features of this tissue were consistent with hemangioblastoma (Figure 4). After some initial hesitation, the patient eventually consented to genetic testing for VHL. This showed no evidence for a germline VHL mutation. Various therapeutic strategies were discussed, including radiotherapy and off label treatment with agents targeting VEGF and VEGFR. The patient is currently considering his treatment options.
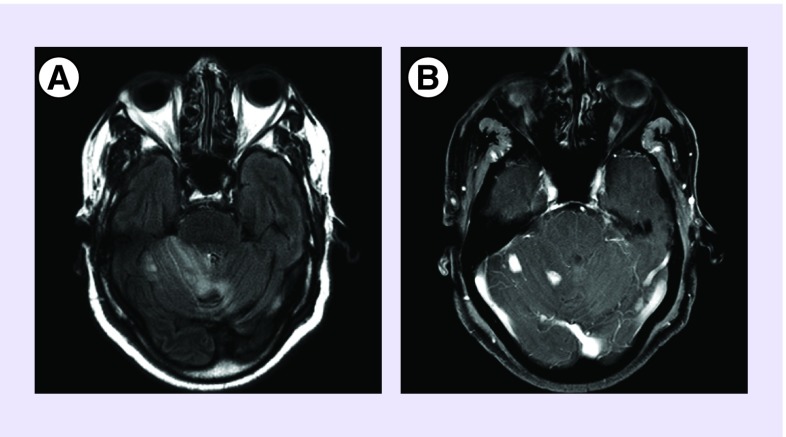
Figure 1. .
Axial fluid-attenuated inversion recovery (A) image showing edema in the upper cerebellum. Axial T1 postcontrast (B) image at the same level showing two enhancing nodules typical of cerebellar hemangioblastomas.
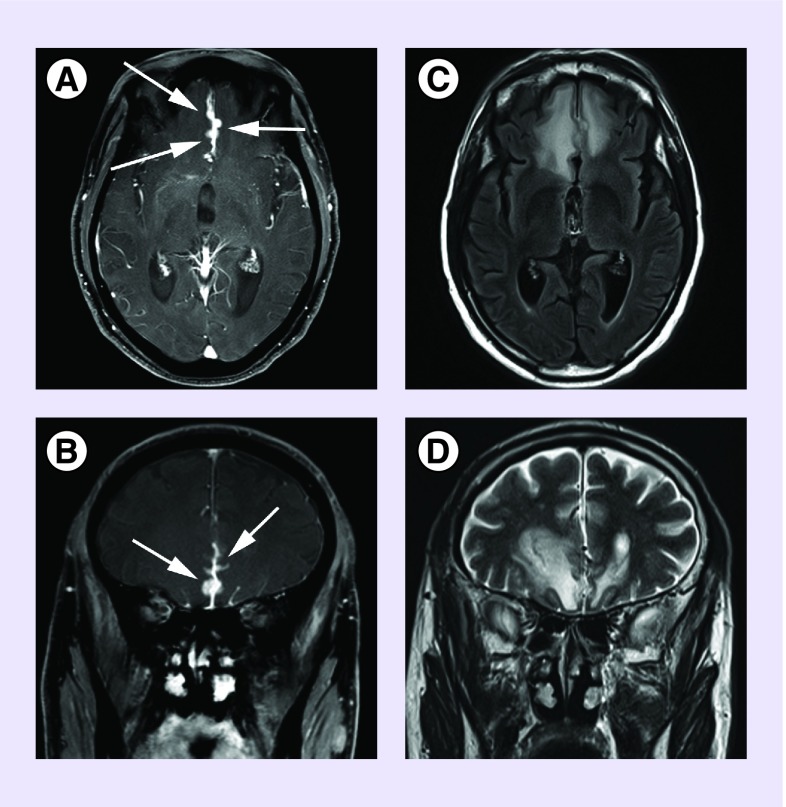
Figure 2. .
Axial (A) and coronal (B) T1 postcontrast images showing dural thickening and tumor studding along the anterior interhemispheric falx (arrows). Axial FLAIR (C) and coronal T2 (D) images showing the extensive edema induced in the adjacent brain parenchyma by the relatively small dural tumors.
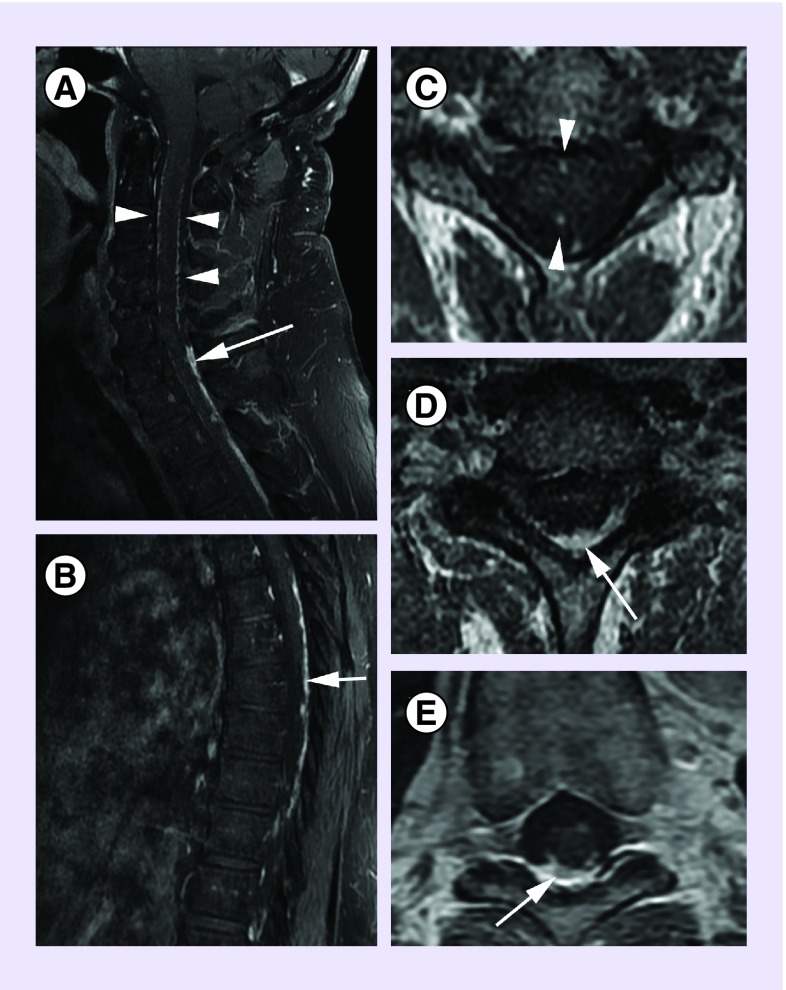
Figure 3. .
Sagittal T1 postcontrast images of the upper spine (A) and of the lower spine (B). Axial T1 postcontrast images at the cervical level (C), the cervicothoracic junction (D) and the mid thoracic level (E). Thin serpiginous enhancement along the dorsal and ventral cord in the cervical region (arrow heads in A & C), represents dilatated vascular structures. Thick and nodular enhancement along the dorsal cord in the lower cervical and thoracic regions (arrows in A–E) represents dural/pial tumor deposits.
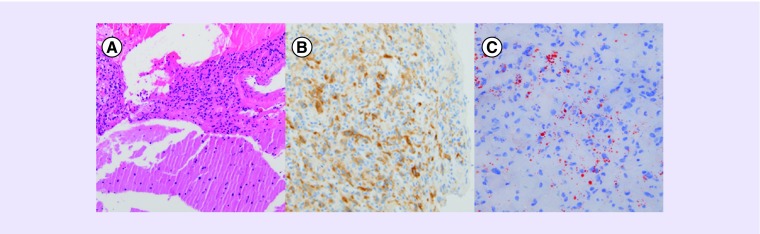
Figure 4. .
(A) The hematoxylin and eosin stained sections show a fragmented specimen comprising normal brain tissue (bottom half) and a moderately cellular proliferation of bland cells (upper half). These findings would fit for an expansile noninfiltrative growth pattern. (B) Immunohistochemical staining reveals reactivity of the lesional cells for inhibin A. (C) Oil-red-O staining on frozen tissue highlights the presence of cytoplasmic lipid droplets in the lesional stromal cells as a feature that is difficult to appreciate on the H&E stained section.
Discussion
CNS hemangioblastomatosis is a distinct but very rare manifestation of hemangioblastoma recurrence. Fewer than 20 individual cases of disseminated disease are reported in the literature [4–6,13–21]. The most typical scenario entails discovery of a relatively small number of tumor deposits along the brain stem and spinal cord, generally years after initial diagnosis and/or treatment of a cerebellar hemangioblastoma. The pattern of diffuse dural tumor caking demonstrated in our case, and in particular, involvement of the supratentorial space, are exceptionally rare features. To our knowledge, only three cases are reported in the literature showing a similarly extensive dissemination of disease with supratentorial involvement, only one of which occurred in a patient without additional features of VHL [5,13,16].
Approximately 60–70% of hemangioblastomas arise sporadically, the rest occurring in association with VHL [22]. Hemangioblastoma accounts for 1–2% of all intracranial neoplasms but for approximately 10% of posterior fossa neoplasms [22,23]. It is the most common primary posterior fossa tumor in adults. Among patients with clinically or genetically proven VHL, hemangioblastoma is the most commonly encountered disease-defining lesion, is usually the first such lesion to present, and will eventually develop in the majority (60–84%) of VHL patients [24–26]. The distinction between sporadic and VHL-associated hemangioblastoma is critical. Hemangioblastoma in a patient carrying a VHL gene mutation is more prone to aggressive behavior than a sporadic hemangioblastoma. Furthermore, patients with a VHL mutation are at increased risk for other potentially malignant tumors, requiring lifelong imaging surveillance of multiple organs.
Although VHL can be diagnosed on the basis of clinical criteria, the gold standard for diagnosis, and certainly for the exclusion of the disease, is through genetic testing for germline VHL gene mutations. After initial reluctance on the part of our patient to undergo genetic testing, he ultimately consented, and his lack of a detectable germline VHL mutation was confirmed. In patients such as ours, who initially present with an isolated hemangioblastoma, no family history of VHL, and no other VHL-defining lesions, the largest study to date estimates the frequency of harboring a VHL germline mutation to be approximately 4% [27]. In addition, of the 4% testing positive for VHL (seven out of 188 subjects), all presented with hemangioblastoma before the age of 40 years. A more recent, although smaller, Norwegian study arrived at a similar, low frequency estimate of approximately 3% [28]. We concede that germline VHL mutation screening will not capture patients who may harbor somatic mosaicism for a VHL mutation, nor those who harbor as yet undiscovered germline mutations. Nevertheless, given the absence of a detectable VHL mutation in our patient, and the fact that he initially presented with his hemangioblastoma at the age of 62 years, a full three decades later than the mean age (29 years) at presentation of VHL-associated hemangioblastoma [2,29], we think it reasonable and appropriate to consider his case as a rare instance of non-VHL hemangioblastomatosis.
Beyond the VHL issue, our case is one of the very few reported examples of disseminated hemangioblastoma involving the supratentorial space. The rarity of supratentorial lesions may reflect the influence of cerebrospinal fluid flow patterns or the effect of gravity on tumor spread. There is little doubt that the pathway for such spread involves access to the subarachnoid spaces. While most of the reported cases of hemangioblastomatosis have occurred in the postoperative setting, it is not clear if this pattern of spread reflects surgical tumor spillage, or an expression, albeit rare, of the tumor's inherent behavior.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Informed consent disclosure
The authors state that they have obtained verbal and written informed consent from the patient/patients for the inclusion of their medical and treatment history within this case report.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Conway JE, Chou D, Clatterbuck RE, Brem H, Long DM, Rigamonti D. Hemangioblastomas of the central nervous system in von Hippel–Lindau syndrome and sporadic disease. Neurosurgery. 2001;48(1):55–62. doi: 10.1097/00006123-200101000-00009. discussion 62–53. [DOI] [PubMed] [Google Scholar]
- 2.Maher ER, Yates JR, Ferguson-Smith MA. Statistical analysis of the two stage mutation model in von Hippel–Lindau disease, and in sporadic cerebellar haemangioblastoma and renal cell carcinoma. J. Med. Genet. 1990;27(5):311–314. doi: 10.1136/jmg.27.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This is a much referenced study investigating the age incidence curves of sporadic versus von Hippel–Lindau-associated hemangioblastoma with results lending support to the two-stage mutation model for tumorigenesis.
- 3.Aldape KD, Plate KH, Vortmeyer AO, Zagzag D, Neuman HPH. Haemangioblastoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. The 2007 WHO Classification of Tumours of the Central Nervous System. International Agency for Research on Cancer; Lyon, France: 2007. pp. 184–186. [Google Scholar]
- 4.Kim HR, Suh YL, Kim JW, Lee JI. Disseminated hemangioblastomatosis of the central nervous system without von Hippel–Lindau disease: a case report. J. Korean Med. Sci. 2009;24(4):755–759. doi: 10.3346/jkms.2009.24.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courcoutsakis NA, Prassopoulos PK, Patronas NJ. Aggressive leptomeningeal hemangioblastomatosis of the central nervous system in a patient with von Hippel–Lindau disease. AJNR Am. J. Neuroradiol. 2009;30(4):758–760. doi: 10.3174/ajnr.A1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung SY, Jeun SS, Park JH. Disseminated hemangioblastoma of the central nervous system without Von Hippel–Lindau disease. Brain Tumor Res. Treat. 2014;2(2):96–101. doi: 10.14791/btrt.2014.2.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Innus C, Patterson J. Hemangioblastoma without von Hippel-Lindau disease. JAAPA. 2007;20(11):31–22. doi: 10.1097/01720610-200711000-00017. 28. [DOI] [PubMed] [Google Scholar]
- 8.Jayaraman MV, Boxerman JL, Atlas SW. Magnetic Resonance Imaging of the Brain and Spine (4th Edition) Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2009. Adult Brain Tumors; pp. 510–515. [Google Scholar]
- 9.Yousem DM, Grossman RI. Neuroradiology: the Requisites (3rd Edition) Mosby/Elsevier; Philadelphia, PA, USA: 2010. Neoplasms of the Brain; pp. 87–89. [Google Scholar]
- 10.Slater A, Moore NR, Huson SM. The natural history of cerebellar hemangioblastomas in von Hippel-Lindau disease. AJNR Am. J. Neuroradiol. 2003;24(8):1570–1574. [PMC free article] [PubMed] [Google Scholar]
- 11.Plaza MJ, Borja MJ, Altman N, Saigal G. Conventional and advanced MRI features of pediatric intracranial tumors: posterior fossa and suprasellar tumors. AJR Am. J Roentgenol. 2013;200(5):1115–1124. doi: 10.2214/AJR.12.9725. [DOI] [PubMed] [Google Scholar]
- 12.Ho VB, Smirniotopoulos JG, Murphy FM, Rushing EJ. Radiologic-pathologic correlation: hemangioblastoma. AJNR Am. J. Neuroradiol. 1992;13(5):1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q, Ma L, Li WY, Chen J, Ju Y, Hui XH. Von Hippel-Lindau disease manifesting disseminated leptomeningeal hemangioblastomatosis: surgery or medication? Acta Neurochirurgica. 2011;153(1):48–52. doi: 10.1007/s00701-010-0827-y. [DOI] [PubMed] [Google Scholar]
- 14.Hanse MC, Vincent A, Van Den Bent MJ. Hemangioblastomatosis in a patient with von Hippel-Lindau disease. J. Neurooncol. 2007;82(2):163–164. doi: 10.1007/s11060-006-9321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lightfoot NJ, Lucas PG, Finnis ND. Disseminated haemangioblastoma without evidence of the von Hippel-Lindau syndrome or haemangioblastomatosis – a case report and clinico-pathological correlation. Clin. Neurol. Neurosurg. 2007;109(3):305–310. doi: 10.1016/j.clineuro.2006.12.007. [DOI] [PubMed] [Google Scholar]; • Presents a patient similar to ours along with a discussion of the natural history of the disease.
- 16.Kato M, Ohe N, Okumura A, et al. Hemangioblastomatosis of the central nervous system without von Hippel-Lindau disease: a case report. J. Neurooncol. 2005;72(3):267–270. doi: 10.1007/s11060-004-2244-7. [DOI] [PubMed] [Google Scholar]
- 17.Mohan J, Brownell B, Oppenheimer DR. Malignant spread of haemangioblastoma: report on two cases. J. Neurol. Neurosurg. Psychiatry. 1976;39(6):515–525. doi: 10.1136/jnnp.39.6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tohyama T, Kubo O, Kusano R, Miura N, Himuro H. [A case of hemangioblastoma with subarachnoid dissemination] No Shinkei Geka. 1990;18(1):83–88. [PubMed] [Google Scholar]
- 19.Reyns N, Assaker R, Louis E, Lejeune JP. Leptomeningeal hemangioblastomatosis in a case of von Hippel-Lindau disease: case report. Neurosurgery. 2003;52(5):1212–1215. discussion 1215–1216. [PubMed] [Google Scholar]
- 20.Akimoto J, Fukuhara H, Suda T, Nagai K, Hashimoto R, Michihiro K. Disseminated cerebellar hemangioblastoma in two patients without von Hippel-Lindau disease. Surg. Neurol. Int. 2014;5:145. doi: 10.4103/2152-7806.142321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weil RJ, Vortmeyer AO, Zhuang Z, et al. Clinical and molecular analysis of disseminated hemangioblastomatosis of the central nervous system in patients without von Hippel-Lindau disease. Report of four cases. J. Neurosurg. 2002;96(4):775–787. doi: 10.3171/jns.2002.96.4.0775. [DOI] [PubMed] [Google Scholar]
- 22.Singounas EG. Haemangioblastomas of the central nervous system. Acta Neurochirurgica. 1978;44(1–2):107–113. doi: 10.1007/BF01401634. [DOI] [PubMed] [Google Scholar]
- 23.Richard S, David P, Marsot-Dupuch K, Giraud S, Béroud C, Resche F. Central nervous system hemangioblastomas, endolymphatic sac tumors, and von Hippel–Lindau disease. Neurosurg. Rev. 2000;23(1):1–22. doi: 10.1007/s101430050024. discussion 23–24. [DOI] [PubMed] [Google Scholar]
- 24.Wanebo JE, Lonser RR, Glenn GM, Oldfield EH. The natural history of hemangioblastomas of the central nervous system in patients with von Hippel-Lindau disease. J. Neurosurg. 2003;98(1):82–94. doi: 10.3171/jns.2003.98.1.0082. [DOI] [PubMed] [Google Scholar]
- 25.Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet. 2003;361(9374):2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 26.Maher ER, Yates JR, Harries R, et al. Clinical features and natural history of von Hippel–Lindau disease. Q. J. Med. 1990;77(283):1151–1163. doi: 10.1093/qjmed/77.2.1151. [DOI] [PubMed] [Google Scholar]
- 27.Woodward ER, Wall K, Forsyth J, Macdonald F, Maher ER. VHL mutation analysis in patients with isolated central nervous system haemangioblastoma. Brain. 2007;130(Pt 3):836–842. doi: 10.1093/brain/awl362. [DOI] [PubMed] [Google Scholar]; •• The largest study to date assessing frequency of von Hippel–Lindau germline mutations in patients presenting with sporadic hemangioblastoma.
- 28.Rønning P, Andresen PA, Hald JK, et al. Low frequency of VHL germline mutations in Norwegian patients presenting with isolated central nervous system hemangioblastomas – a population-based study. Acta Neurol. Scand. 2010;122(2):124–131. doi: 10.1111/j.1600-0404.2009.01274.x. [DOI] [PubMed] [Google Scholar]
- 29.Neumann HP, Eggert HR, Weigel K, Friedburg H, Wiestler OD, Schollmeyer P. Hemangioblastomas of the central nervous system. A 10-year study with special reference to von Hippel–Lindau syndrome. J. Neurosurg. 1989;70(1):24–30. doi: 10.3171/jns.1989.70.1.0024. [DOI] [PubMed] [Google Scholar]