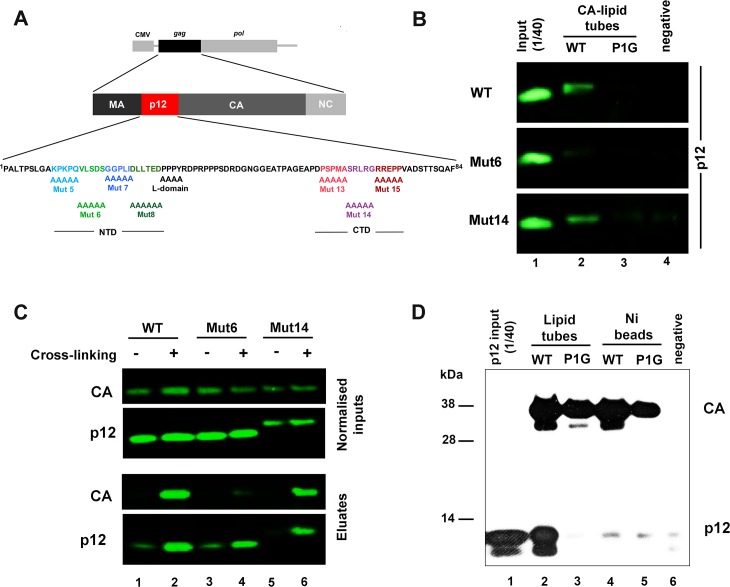
Fig 1. The N-terminal domain of MLV p12 directly binds hexameric CA arrays.
(A) Schematic representation of the MLV Gag-Pol expression plasmid used in this study, showing the Mo-MLV p12 sequence with alanine substitution mutants below. (B) Immunoblot showing binding of p12 to N-MLV CA. His-tagged WT (lane 2) or P1G mutant (lane 3) CA proteins were immobilised on lipid nanotubes comprising the Ni2+-chelating lipid, DGS-NTA, prior to incubation with purified p12_WT, p12_mut6 (NTD mutant) or p12_mut14 (CTD mutant). No lipid tubes or CA were included in the negative control reactions (lane 4). CA nanotubes were pelleted, and any bound p12 was revealed by SDS-PAGE and immunoblotting with a rabbit anti-p12 antibody. 1/40th of the purified p12 protein input was loaded in lane 1. (C) Co-immunoprecipitation (Co-IP) of CA with p12 from viral lysates. Mo-MLV virus-like particles (VLPs) carrying myc-tagged p12_WT (lanes 1 and 2), p12_mut6 (lanes 3 and 4) or p12_mut14 (lanes 5 and 6) were either untreated (lanes 1, 3 and 5) or fixed in 1% formaldehyde (lanes 2, 4 and 6) prior to lysis. Myc-p12 was immunoprecipitated from normalised viral lysates using an anti-myc antibody. 1/50th of input lysates and 1/5th of Co-IP elutions were analysed by immunoblotting with anti-CA and rabbit anti-p12 antibodies. (D) Immunoblot showing binding of p12 specifically to CA hexamers. His-tagged WT (lanes 2 and 4) or P1G mutant (lanes 3 and 5) CA proteins were immobilised as hexamers on lipid nanotubes (lanes 2 and 3), or randomly on Ni-NTA beads (lanes 4 and 5). No CA, beads or tubes were included in the negative control (lane 6). After incubation with p12, the CA assemblies were pelleted and analysed by SDS-PAGE and immunoblotting for CA (anti-His) and p12 (mouse anti-p12). 1/40th of the purified p12 protein input was loaded in lane 1.