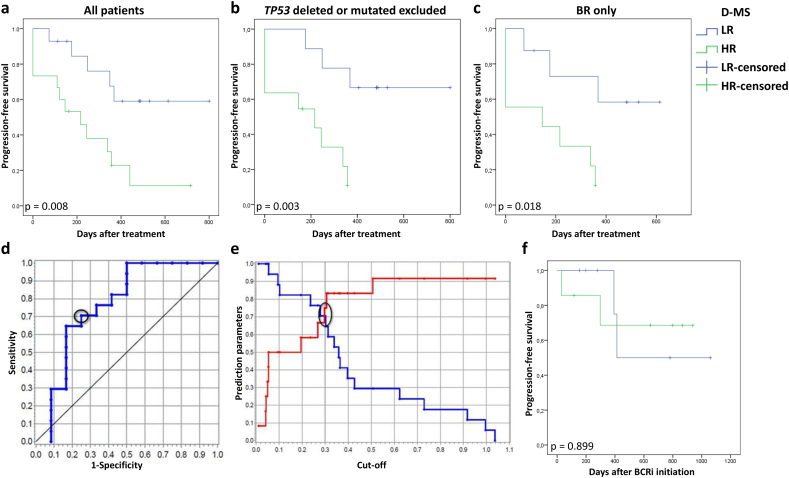
Fig. 4.
Progression-free survival after treatment with chemo-immunotherapy according to dichotomized metabolic score (D-MS). Analysis of all CLL patients treated with chemo-immunotherapy (HR n = 15, LR n = 14; A). Subgroup analysis after exclusion of patients that are TP53 deleted or mutated (HR n = 11, LR n = 9, B). Subgroup analysis of patients treated with the combination of bendamustine with rituximab (BR; HR n = 9, LR n = 8; C). Based on the results of CLL patient sample analysis, the cut-off of the test value |MS-2| was variated in the sense of an ROC analysis (HR n = 15, LR n = 14). It was ascertained that the sensitivity and the specificity were similar at a value of 0.3 correlating about 75% (D). The discriminatory performance of the test value |MS-2| is shown in the ROC curve with an area under curve of 0.77 (HR n = 15, LR n = 14; E). PFS was determined in CLL patients who were treated with BCRi after sampling (n = 14, ibrutinib/idelalisib HR n = 5/2, LR n = 3/4; F).