Abstract
Trimethoprim, a commonly used antibacterial agent, is widely applied in the treatment of variety of infections in human. A few studies have demonstrated an extensive exposure of man to antibiotics, but there is still a lack of data for cytotoxic effects including nephrotoxicity, gastrointestinal toxicity, hematotoxicity, neurotoxicity and ototoxicity. The main purpose behind this study was to determine cytotoxic and genotoxic activities of trimethoprim (1), trimethoprim with maleic acid (2) and trimethoprim in conjugation with oxalic acid dihydrate (3). The cytotoxic effects of these three conjugates were elucidated by employing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoium bromide (MTT) assay using embryonic rat fibroblast-like cell line (F2408) and H-ras oncogene activated embryonic rat fibroblast-like cancer cell line (5RP7). Additionally, determination of genotoxic activity of these three compounds were studied by using cytokinesis blocked micronucleus assay (CBMN) in human lymphocytes. The results demonstrated that trimethoprim alone and its combination with other compounds are able to induce both cytotoxic and genotoxic damage on cultured cells (F2408, 5RP7, human lymphocytes).
Keywords: Trimethoprim, Maleic acid, Oxalic acid dihydrate, Cytotoxicity, Genotoxicity
Introduction
Trimethoprim (2,4,-diamino-5-(3′,4′,5′-trimethoxybenzyl)-pyrimidine, TMP) is a member of the synthetic 2,4-diaminopyrimidine family. It is one of the earliest antibacterial diaminopyrimidine introduced to clinical use. It has a broad-spectrum for the treatment of numerous infections especially urinary tract infections (UTI) caused by susceptible bacteria such as Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, Enterobacter species, and coagulase-negative Staphylococcus species, including S. saprophyticus (Wang et al. 2010; Smilack 1999). It is still widely used as a sulphonamide potentiator in medicine (Lampert and O’Grady 1992). It is known that trimethoprim-sulfamethoxazole (TMP-SMX) is a commonly used antibiotic, partially because of its low cost and easy availability (Stuhec 2014; Smilack 1999). The antibacterial activity of the combination of sulfamethoxazole (SMX) with TMP is more efficient than TMP alone due to the sequential inhibition of the bacterial synthesis of tetrahydrofolic acid by disrupting nucleic bases and acids synthesis. These outcomes prompted researchers to investigate the molecular nature of dihydrofolate reductase inhibition induced by the drug (Bastien et al 2012). It is now well understood that folate deficiency can play crucial role in the genetic process (Chen et al. 2013; Otto and Sicinski 2017). Changes in dihydrofolate reductase inhibition play also an important role in carcinogenesis (Hui-Li et al. 2017; Wang and Huang 2017; Osborn et al. 1958). In cancer research, there has been a continuous effort to determine the best active pharmaceutical ingredient to improve treatment of disease. To make the drugs readily applicable in the cancer field, scientists are still on the way to develop new drug/conjugates with high potency under low adverse effect.
Consequently, in the literature there are various studies about the in-vivo and in-vitro activities of TMP. Abou-Eisha et al. evaluated genotoxic properties of TMP for its effects in terms of nuclear DNA damage in cultured peripheral blood lymphocytes in terms of chromosome and DNA alterations (Abou-Eisha 2006).
The genotoxicity of diaveridine and TMP was investigated in bacterial umu test, in bacterial reverse mutation test, in in vitro chromosome aberration test, in in vivo rodent bone marrow micronucleus test, and in in vivo comet assay, in five mouse organs. Both compounds were negative in the umu test. TMP, with and without S9 mix, did not exhibit clastogenic activity. No clastogenic activity in CHL cells was detected for TMP. TMP treatment was not followed by a significant increase in migration values of DNA (Ono et al. 1997). Liguoro et al. studied TMP’s sublethal effects in four freshwater organisms (Liguoro et al. 2012). TMP showed varying levels of toxicity in the four tests performed. Another study aimed to understand the ozonation process of TMP in aqueous solutions. This study proved the dominant role of ozone rather than hydroxyl radicals in the reaction along with the potential risk after ozonation. The results indicate that one of the most sensitive species as an increased toxicity after ozonation of TMP was observed (Kuang et al. 2013).
MN formations which originate from chromosomal fragments or whole chromosomes in peripheral blood lymphocytes can be used as a cytogenetic marker to detect the effects of DNA damaging agents in the prediction of cancer (Fenech 2000; Norppa 2004). It is known that MN provide a measure for both chromosome breakage and chromosome loss, and it has been shown that it is also a sensitive indicator of chromosome damage (Fenech et al. 1999). Therefore, we decided to investigate these conjugates’ genotoxic potential with the CBMN test in human lymphocyte culture in vitro.
Resistant bacterial species have appeared and have spread all over the world because of incredible genetic elasticity of the microorganisms. Some recent papers reported that widespread use of TMP led to drug resistance in bacterial species (Abou-Eisha et al. 1999; Huovinen et al. 1995; Baccanari 1995). Apart from TMP’s antibacterial effects on humans further investigations into its mechanism of action has to be done.
Similar to SMX the therapeutic efficacy of drug can be maximized by combining TMP with some organic or inorganic salts in terms of increasing solubility and plasma half life of the drug in the body. Following this trend, some recent papers reported that combination of TMP with organic/inorganic salts can significantly improve drug performance (Tunalı et al. 2011).
Actually, TMP has a basic nature (pKa 7.3) and after oral administration, nearly 60% of the dose of TMP is excreted as an unmodified state (Sweetman 2005). To optimize the antibacterial effect an acidifying agent such as hydrochloride generally accompanies with the administration of drugs. A salt form can improve gastrointestinal tolerance without interfering with oral absorption considering the fluctuating pH experienced by the drug molecule orally administered. Around 50% of the basic drugs is in their hydrochloride salt forms. On the other hand, hydrochloride salt forms usually cause high acidity in formulations and have a high risk of facilitating irritations. Due to the limitations of hydrochloride, alternative counter ions have been explored (Elshaer et al. 2012). Alternatively, TMP may be incorporated with any organic acid such as lactic acid, citric acid, tartaric acid, maleic acid, malic acid, glutamic acid and aspartic acid in order to promote absorption, the rate of absorption and to facilitate biocompatibility of TMP. According to a literature example, sulfate, citrate, lactate, tartrate, malate and maleate compounds were coupled with TMP (Tunalı et al. 2011) and the conjugates were found to be more potent than the drug used alone. In this case, Tunalı et al. showed the antibacterial and antifungal activities of these TMP conjugates but the effects of these compounds on mammalian cells have not been investigated so far.
In this manuscript, TMP was conjugeted with maleic acid and oxalic acid dihydrate leading to the corresponding drug conjugates. The efficacy and in-vitro genotoxic/cytotoxic activities of trimethoprim (Wang et al. 2010) alone, trimethoprim with maleic acid (Smilack 1999) and trimethoprim with oxalic acid dehydrate (Lampert and O’Grady 1992) conjugates were investigated. These conjugates are expected to provide the future therapeutic options for treatment of infections with TMP-resistant bacteria species disposing of analogues among the growth promoters. Some studies exist in literature concerning the antimicrobial, antibacterial activity and genotoxicity of TMP, however, there is no report on genotoxicity and cytotoxicity of TMP and/or its conjugates.
Materials and methods
Chemistry
Trimethoprim (TMP), oxalic acid dihydrate and maleic acid were purchased from Sigma (Istanbul, Turkey). All other reagents were used as purchased from commercial suppliers without further purification.
In-vitro genotoxicity assay (CBMN)
For identification of health risk from exposure to mutagens, scientists investigate sensitive biomarkers which are specific to the detection of exposures (Au 2003). The cytokinesis-block micronucleus (CBMN) assay, which is a commonly used method for measuring DNA damage based on cytokinesis inhibition by cytochalasin B (Cyt-B), leads to micronuclei in (binucleate) cells that have completed their first in vitro division after the treatment with the test agent. The key advantages of the CBMN assay are the detection of both clastogenic and aneugenic events and the identification of cells which divided once in culture (Leng et al. 2009).
The micronucleus test was performed as described by Fenech (2000). Peripheral blood lymphocytes were taken from two nonsmoking healthy donors between the ages of 25 and 30 years. Whole blood was added to 2.5 ml Chromosome Medium B and incubated at 37 °C for 72 h. TMP and its conjugates were dissolved in distilled water and test concentrations were prepared as 50 and 25 µg/ml. The cells were treated with TMP, TMP + maleic acid and TMP + oxalic acid dihydrate (50 and 25 µg/ml) for 24 and 48 h. A negative and a positive control (Mitomycin-C) were also added. Cytocalasin B was added to the cultures at a final concentration of 6 µg/ml 44 h after the initiation of the cultures. After 72 h the cells were harvested, treated with hypotonic solution (% 0.4 KCI), and were fixed three times with fixative (methanol: glacial acetic acid). The slides were air dried and stained with 5% Giemsa. The frequency of micronucleus was determined by analyzing 1000 binucleated cells for each donor and treatment. Statistical differences between MN number of treated cells and MN number of their solvent controls were evaluated with Dunnett test in ANOVA. The cell proliferation index (CPI) was calculated as follows: (B + 2P)/(M + B + P) where M, B, P, respectively, are the number of cells that have not yet entered the first mitosis (M, mononucleated), the number of cells that have divided once (B, binucleated) and twice (P, plurinucleated), respectively. (M + P + P) represents a total of at least 1000 cells scored.
In-vitro cytotoxicity assay (MTT)
The cytotoxicity effect of test compound on healty rat embryo fibroblast-like cell line (F2408) and H-ras oncogene activated rat embryo fibroblast-like cancer cell line (5RP7) was evaluated by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay according to the method described by Mosmann (1983). Briefly, F2408 and 5RP7 cells were seeded in a flat bottomed 96-well plate (Techno Plastic Products AG) at a density of 5 × 103 cells/well in DMEM containing 10% FBS. The plate was incubated at 37 °C with 5% CO2 for 24 h, and then the test compounds were prepared and added to reach a final concentration of 50, 100 and 200 μM for all TMP containing samples and 5, 10, 20, 40 and 50 μM concentrations for cisplatin samples, respectively. Eight replicate wells per concentration were used and repeated in triplicate at different intervals. Untreated medium controls (blank) and solvent controls (DMSO at a final concentration of 0.1%, v/v) were also assayed in parallel. Cells were further incubated for 24, 48 and 72 h at 37 °C with 5% CO2; then, the medium was replaced with DMEM containing 10% FBS. 10 μl of filter sterilized MTT solution (5 mg/ml in PBS) was added to each well and further incubated at 37 °C with 5% CO2 for 2 h. At the end the incubation medium was aspirated from the wells and 100 μl of DMSO was added to dissolve the insoluble formosan crystals formed. The absorbance was measured at 570 nm using a microtiter plate reader (Bio-Tek, ELX808IU, Winooski, VT, USA). Cisplatin was used as positive control, which was also initially dissolved in DMSO. The relative % cell viability was calculated from the following equation: Relative percent cell viability = (Atest/Acontrol) × 100%. (A test is the absorbance of the sample treated cells and A control is the absorbance of the untreated cells. Each absorbance was taken to be the mean of triplicate measurements). The cell viability was represented as a percentage relative to untreated cells as a control.
Statistical analysis
The Statistical Package for Social Sciences (SPSS) software has been used for the statistical analyses of assessment of the MTT assay. Data were evaluated through the use of one-way ANOVA (Analysis of Variance) followed by the Tukey test. Differences between two means were considered statistically significant at p < 0.05 and are indicated with asterisks (*). Data in experiments are expressed as the mean ± standard deviation.
Results
TMP alone, TMP with maleic acid and TMP with oxalic acid dihydrate were evaluated for their cytotoxic and genotoxic activities on healhty lymphocytes cultures and cancer cell lines. The human lymphocytes were treated with 50 and 25 µg/ml concentrations of TMP and its conjugates dissolved in distilled water, for 24 and 48-h treatment periods to investigate the effects of these compounds on MNs in human lymphocytes.
The effects of trimethoprim (TMP) and its conjugates on MN formation in human peripheral blood lymphocytes are summarized in Table 1. Our results showed that the use of TMP alone caused significant MN formations at all concentrations of all treatment periods when compared to negative control on peripheral lymphocytes. The frequencies of formations of MN caused by the TMP + oxalic acid dihydrate at a 50 µg/ml dose was found to be statistically significant at 24-h (p < 0.05) and 48-h (p < 0.01). TMP + maleic acid was statistically the most genotoxic conjugate at 48-h.(p < 0.001). In addition, TMP and its conjugates did not decrease the CPI values for both treatment periods and at all the concentrations.
Table 1.
The frequency of micronucleus (MN) and Cell proliferation index (CPI) of cultured human lymphocytes treated with TMP, TMP + maleic acid, TMP + oxalic acid dihydtrate
| Test substances | Treatment time (h) | Concentration (µg/ml) | MN ± SD | CPI ± SD |
|---|---|---|---|---|
| Negative Control | 24 | - | 7.5 ± 0.40 | 0.70 ± 0.07 |
| MMC | 24 | 0.3 | 21.5 ± 0,50*** | 0.31 ± 0.14*** |
| TMP | 24 | 50 | 13.0 ± 1.41** | 0.37 ± 0.06 |
| 24 | 25 | 11.5 ± 2.12* | 0.44 ± 0.04 | |
| TMP + maleic acid | 24 | 50 | 16.4 ± 050** | 0.42 ± 0.05 |
| 24 | 25 | 9.5 ± 2.12* | 0.50 ± 0.07 | |
| TMP + oxalic acid dihydtrate | 24 | 50 | 11.5 ± 0.60* | 0.52 ± 0.09 |
| 24 | 25 | 9.0 ± 0.46 | 0.55 ± 0.13 | |
| Negative Control | 48 | - | 8.0 ± 0.35 | 0.68 ± 0.09 |
| MMC | 48 | 0.3 | 25.5 ± 0.44*** | 0.26 ± 0.05*** |
| TMP | 48 | 50 | 19.0 ± 1.62*** | 0.34 ± 0.13 |
| 48 | 25 | 14.5 ± 0.70** | 0.35 ± 0.16 | |
| TMP + maleic acid | 48 | 50 | 20.5 ± 0.70*** | 0.33 ± 0.02 |
| 48 | 25 | 18.5 ± 0.45*** | 0.47 ± 0.05 | |
| TMP + oxalic acid dihydtrate | 48 | 50 | 17 ± 0.70** | 0.53 ± 0.07 |
| 48 | 25 | 12 ± 0.44 * | 0.57 ± 0.04 |
MMC mitomycin-C, CPI cell proliferation index, TMP trimethoprim, ± SD standard deviation values
*Significantly different from the control p < 0.05 (t-test)
**Significantly different from the control p < 0.01 (t-test)
***Significantly different from the control p < 0.001 (t-test)
On the other hand, cytotoxic effects of trimethoprim and its conjugates were determined on rat embryonic fibroblast-like cells (F2408) and H-ras oncogene-activated rat embryonic fibroblast-like cancer cells (5RP7) by MTT assay. 50, 100 and 200 µM concentrations of TMP and its conjugates were prepared to determine the cytotoxicity of these compounds on F2408 and 5RP7 cells using the MTT (3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. For comparison purposes, the cytotoxicity of cisplatin was evaluated under the same experimental conditions. All compounds demonstrated dose-dependent cytotoxicity against both cell lines, especially against cancer cells. The results are given in Fig. 1.
Fig. 1.
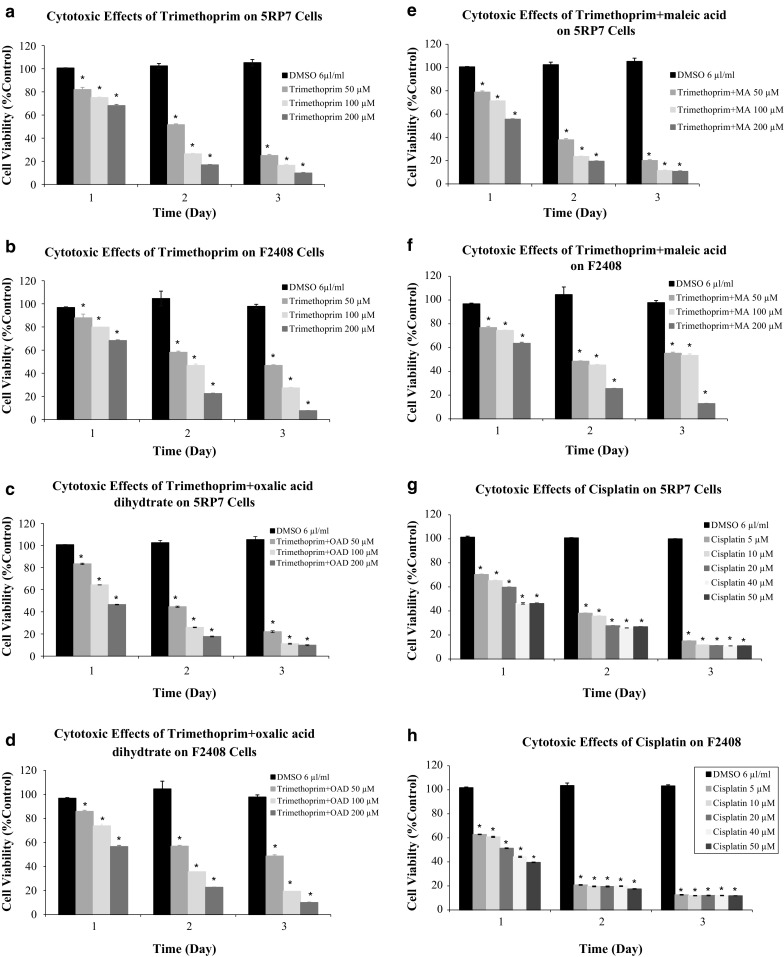
(a–h) Comparisons between cytotoxic activity of TMP, TMP + MA (maleic acid), TMP + OAD (oxalic acid dehydrate) and cisplatin on 5RP7 (a–c–e–g) and on F2408 (b–d–f–h) cell lines are determined in the MTT assay. Dose-response curves of the anti-proliferative effect of compounds for MTT assays are performed after 24, 48 and 72 h of exposures. The results are expressed as the mean ± SD. * indicating significant difference from the control group by the Tukey test (p < 0.05). Cisplatin was used as a positive control
Discussion
In the present study, we evaluated the genotoxic potential of TMP and its conjugates using CBMN assay as the genetic endpoints in human peripheral lymphocytes and the cytotoxic effects of these compounds were evaluated on rat embryonic fibroblast-like cells (F2408) and H-ras oncogene-activated rat embryonic fibroblast-like cancer cells (5RP7) using the MTT assay. Dose-dependent increase of MNs showed that TMP and its conjugates most probably possess a genotoxic risk to human peripheral lymphocytes. MN frequencies observed in cultures treated with TMP and its conjugates were significantly higher than the control values.
Since, there is no adequate study with TMP’s conjugates, the human risk of them could not be evaluated. Thus, taking into account this lack of information about the genotoxic potential of TMP’s conjugates in human lymhocytes, we investigated the cytogenetic activity of this drug in in vitro cultured human lymphocyte. The results have been supported by the study of Abou-Eisha et al. with TMP (Abou-Eisha 2006, 1999). They showed that TMP has a significant clastogenic potential, as detected by a dose-dependent increase of the incidence of micronuclei as and significantly greater than control levels at the highest concentrations. Furthermore, TMP was investigated in a study for its genotoxic effect in human lymphocytes and it had been seen that it increased MN and SCE frequency which are markers of genotoxic damage in human lymphocytes (Abou-Eisha et al. 1999). Our results have also been supported by this study for genotoxic potential of TMP’s conjugates and clearly indicated that these conjugates and their doses have genotoxic activity for a very short time in lymphocytes; the MN frequency in binucleated cells was its indicator. Furthermore, TMP was positive for induction of CA and sister-chromatid exchanges (SCE) frequencies in Chinese hamster ovary (CHO) cells treated at doses that caused cell cycle delay with and without metabolic activity (Galloway et al. 1985; Rosenkranz et al. 1990). In a study, the toxicity of sulfamethazine and its additivity to other veterinary sulfonamides and TMP were evaluated in Daphnia manga. The acute EC50 was in the range 131–270 mg L1 for all compounds tested with the exception of sulfaguanidine (Liguoroa et al. 2009). TMP–SMX was studied for hematologic disorders in patients. After exposure to TMP–SMX, patients developed severe thrombocytopenia and leucopenia. The results showed that TMP–SMX combination was not well tolerated, even for prolonged periods (Kocak et al. 2006). The activities of TMP and some sulfonamides were examined against developing Sarcocystis neurona merozoites in bovine turbinate cell cultures. None of the agents tested alone or in combination were toxic for the BT cells (Lindsaya and Dubey 1999). Stuhec reported that TMP-SMX induced hallucinations in immunocompetent patients (Stuhec 2014). Caselli et al. (2014) showed that a single-day course of prophylaxis with TMP–SMX may be sufficient to prevent Pneumocystis (jirovecii [carinii]) pneumonia (PCP) in children with cancer undergoing intensive chemotherapy regimens.
According to our results; trimethoprim and its conjugates significantly increased micronucleus frequency in a time dependent fashion, in human lymphocytes. When we compared these compounds, TMP + maleic acid was the more effective genotoxic conjugate than TMP + oxalic acid dihydrate at all treatment periods and doses. Also, TMP and TMP + maleic acid at maximum dose of 50 µg/ml provided similar results after statistical analysis, evaluated by Dunnett test in ANOVA. Therefore, we reported that these compounds had genotoxic effects with the in vitro CBMN technique, in human peripheral blood lymphocytes. When we considered the CPI results, no meaningful reduction in the cell proliferation was observed.
In addition, cytotoxicity was assessed in this study by MTT assay. For TMP, the lowest concentrations (50 µM) exhibited no significant cytotoxicity on the first day. But concentrations of 100 and 200 µM caused inhibition of cell proliferation by around 50%, and more on the second day. Results showed that TMP caused similar cytotoxic effects on both 5RP7 and F2408 cells. For TMP + maleic acid at the lowest concentration (50 µM) caused inhibition of F2408 cell proliferation by 23%, 51.5%, and by 44.75% while the same concentration of this compound caused inhibition of 5RP7 cell prolifertion by 25.6, 54.7, and 46.7%, 24 h, 48 h and 72 h after exposure, respectively. At the same time, after 24 h of incubation with TMP + oxalic acid at a concentration of 100 µM, the viability was 74% for F2408 cells, and 64% for 5RP7 cells. The MTT assay showed a sharp decreased on mitochondrial activity in 5RP7 cells treated with the concentration of 100 µM, at end of the second and third day. For instance; the percentage of living healthy cells (F2408) was 45%, whereas that of cancer cells (5RP7) was 24% on the second day. Furthermore, 72 h after treatment with TMP + maleic acid, there was almost no mitochondrial activity determined by the MTT assay for 5RP7 cells any more. 50% of 5RP7 and 36% of F2408 cells were killed with the highest concentration (200 µM) of TMP + maleic acid on the first day. All concentrations of TMP + oxalic acid dihydtrate exhibited significant cytotoxicity on both cells. IC50 value was approximately 200 µM on the first day, and 50 µM on the second day. Cisplatin was found more effective on healthy cells for all concentrations then on cancer cells. Statistical analysis to determine the dose-dependent effects of test materials on mitochondria revealed that, toxicity percentage of all TMP concentrations was significant at 24, 48 and 72 h when compared to untreated control (p < 0.001). TMP + maleic acid combination has the most effective cytotoxic effects on 5RP7 cells among other TMP combinations and TMP.
In conclusion, the results of our study indicated that TMP and its conjugates were found to be genotoxic due to increase of MN frequency in human lymphocytes. TMP and its conjugates have significant cytotoxic effects in 5RP7 and F2408 cells and genotoxic effects in human lymphocyte cultures according to our study. We suppose that these drugs are promising for cancer patients, due to the fact that combination of TMP with some organic acid has more effective cytotoxic activity on cancer cells then on healthy cells. However, further studies are needed.
Conclusions
In summary, In this study we show cytotoxicity of TMP alone and its combination with maleic acid and oxalic acid dihydrate against normal diploid (F2408) and cancer (5RP7) cells and also genotoxicity of these compounds in human peripheral lymphocyte culture. The experimental results validate that the drug conjugates exhibit higher cytotoxic and genotoxic efficacy than TMP alone. Such synergistic combination in medicine can afford a potential strategy to induce excellent cancer cell apoptosis and improve antitumor efficiency.
Acknowledgements
The authors are grateful to Anadolu University, Faculty of Sciences, Department of Biology and Bezmialem Vakif University, Faculty of Pharmacy, Department of Pharmaceutical Chemistry. Also the authors thank to Tuba Ertekin and Barlas Benkli.
Contributor Information
Devrim Güzel Bayülken, Phone: +90 222 3350580, Email: dvrmguzel@gmail.com.
R. Beklem Bostancıoğlu, Email: beklemb@gmail.com.
A. Tansu Koparal, Email: akoparal@anadolu.edu.tr.
Berrin Ayaz Tüylü, Email: bayaz@anadolu.edu.tr.
Aydan Dağ, Email: adag@bezmialem.edu.tr.
Kadriye Benkli, Email: kbenkli@bezmialem.edu.tr.
References
- Abou-Eisha A. Evaluation of cytogenetic and DNA damage induced by the antibacterial drug, trimethoprim. Toxicol in vitro. 2006;20:601–607. doi: 10.1016/j.tiv.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Abou-Eisha A, Creus A, Marcos R. Genotoxic evaluation of the antimicrobial drug, trimethoprim, in cultured human lymphocytes. Mutat Res. 1999;440:157–162. doi: 10.1016/S1383-5718(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Au WW. Mutagen sensitivity assays in population studies. Mutat Res Rev Mutat Res. 2003;544:273–277. doi: 10.1016/j.mrrev.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Baccanari DP. Trimethoprim-sulfamethoxazole. In: Munson PL, Mueller RA, Breese GR, editors. Principles of pharmacology: basic concepts and clinical applications. New York: Chapman & Hall; 1995. pp. 1301–1317. [Google Scholar]
- Bastien D, Ebert MCCJC, Delphine F, Toulouse J, Kadnikova N, et al. Fragment-based design of symmetrical bis-benzimidazoles as selective inhibitors of the trimethoprim-resistant, type II R67 dihydrofolate reductase. J Med Chem. 2012;55:3182–3192. doi: 10.1021/jm201645r. [DOI] [PubMed] [Google Scholar]
- Caselli D, Petris MG, Rondelli R, Carraro F, Colombini A, et al. Single-day trimethoprim/sulfamethoxazole prophylaxis for pneumocystis pneumonia in children with cancer. J Pediatr. 2014;164:389–392. doi: 10.1016/j.jpeds.2013.10.021. [DOI] [PubMed] [Google Scholar]
- Chen C, Ke J, Zhou XE, Yi W, Brunzelle JS, et al. Structural basis for molecular recognition of folic acid by folate receptors. Nature. 2013;500:486–489. doi: 10.1038/nature12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshaer A, Hanson P, Worthington T, Lambert P, Mohammed AR. Preparation and characterization of amino acids-based trimethoprim salts. Pharmaceutics. 2012;4:179–196. doi: 10.3390/pharmaceutics4010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;455:81–95. doi: 10.1016/S0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- Fenech M, Holland N, Chang WP, Zeiger E, Bonassi S. The HUman MicroNucleus Project—an international collaborative study on the use of the micronucleus technique for measuring DNA damage in humans. Mutat Res. 1999;428:271–283. doi: 10.1016/S1383-5742(99)00053-8. [DOI] [PubMed] [Google Scholar]
- Galloway SM, Bloom AD, Resnick M, Margolin BH, Nakamura F, et al. Development of a standard protocol for in vitro cytogenetic testing with Chinese hamster ovary cells: comparison of results for 22 compounds in two laboratories. Environ Mutagen. 1985;7:1–51. doi: 10.1002/em.2860070102. [DOI] [PubMed] [Google Scholar]
- Hui-Li N, Xiang M, Eng-Hui C, Chui WK. Design, synthesis, and biological evaluation of coupled bioactive scaffolds as potential anticancer agents for dual targeting of dihydrofolate reductase and thioredoxin reductase. J Med Chem. 2017;60:1734–1745. doi: 10.1021/acs.jmedchem.6b01253. [DOI] [PubMed] [Google Scholar]
- Huovinen P, Sundstrom L, Swedberg G, Skold O. Trimethoprim and sulfonamide resistance. Antimicrob Agents Chemother. 1995;39:279–289. doi: 10.1128/AAC.39.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocak Z, Hatipoglu AC, Ertem G, Kinikli S, Tufan A, et al. Trimethoprim sulfamethoxazole induced rash and fatal hematologic disorders. J Infect. 2006;52:49–52. doi: 10.1016/j.jinf.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Kuang J, Huang J, Wang B, Cao Q, Deng S, et al. Ozonation of trimethoprim in aqueous solution: identification of reaction products and their toxicity. Water Res. 2013;47:2863–2872. doi: 10.1016/j.watres.2013.02.048. [DOI] [PubMed] [Google Scholar]
- Lampert HP, O’Grady FW. Diaminopyrimidines. Antibiotic and chemotherapy. Edinburgh: Churchill Livingstone; 1992. pp. 148–153. [Google Scholar]
- Leng HS, Pan Z, Dai Y, Niu Y, Huang C, Bin P, Wang Y, Liu Q, Chen W, Zheng Y. Biomarkers measured by cytokinesis-block micronucleus cytome assay for evaluating genetic damages induced by polycyclic aromatic hydrocarbons. Mutat Res. 2009;677:93–99. doi: 10.1016/j.mrgentox.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Liguoro MD, Leva VD, Bona MD, Merlanti R, Caporale G, et al. Sublethal effects of trimethoprim on four freshwater organisms. Ecotoxicol Environ Saf. 2012;82:114–121. doi: 10.1016/j.ecoenv.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Liguoroa MD, Fioretto B, Poltronieri C, Gallina G. The toxicity of sulfamethazine to Daphnia magna and its additivity to other veterinary sulfonamides and trimethoprim. Chemosphere. 2009;75:1519–1524. doi: 10.1016/j.chemosphere.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Lindsaya DS, Dubey JP. Determination of the activity of pyrimethamine, trimethoprim, sulfonamides, and combinations of pyrimethamine and sulfonamides against Sarcocystis neurona in cell cultures. Vet Parasitol. 1999;82:205–210. doi: 10.1016/S0304-4017(99)00020-5. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Norppa H. Cytogenetic biomarkers and genetic polymorphisms. Toxicol Lett. 2004;149:309–334. doi: 10.1016/j.toxlet.2003.12.042. [DOI] [PubMed] [Google Scholar]
- Ono T, Sekiya T, Takahashi Y, Sasaki YF, Izumiyama F. The genotoxicity of diaveridine and trimethoprim. Environ Toxicol Pharmacol. 1997;3:297–306. doi: 10.1016/S1382-6689(97)00026-4. [DOI] [PubMed] [Google Scholar]
- Osborn MJ, Freeman M, Huennekens FM. Inhibition of dihydrofolic reductase by aminopterin and amethopterin. Proc Soc Exp Biol Med. 1958;97:429–431. doi: 10.3181/00379727-97-23764. [DOI] [PubMed] [Google Scholar]
- Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17:93–115. doi: 10.1038/nrc.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz HS, Ennever FK, Dimayuga M, Klopman G. Significant differences in the structural basis of the induction of sister chromatid exchanges and chromosomal aberrations in Chinese hamster ovary cells. Environ Mol Mutagen. 1990;16:149–177. doi: 10.1002/em.2850160304. [DOI] [PubMed] [Google Scholar]
- Smilack JD. Trimethoprim-sulfamethoxazole. Mayo Clin Proc. 1999;74:730–734. doi: 10.4065/74.7.730. [DOI] [PubMed] [Google Scholar]
- Stuhec M. Trimethoprim-sulfamethoxazole-related hallucinations. Gen Hosp Psychiatry. 2014;36:230.e7-8. doi: 10.1016/j.genhosppsych.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Sweetman SC. Martindale: the complete drug reference. London: Pharmaceutical Press; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunalı Y, Karaca H, İşcan G, Demirci F, Benkli K. Antibacterial and antifungal activities of some trimethoprim salts. Med Chem Res. 2011;21:932–935. doi: 10.1007/s00044-011-9605-5. [DOI] [Google Scholar]
- Wang C, Huang S. Drug development against metastatic cancers. Yale J Biol Med. 2017;90:119–123. [PMC free article] [PubMed] [Google Scholar]
- Wang XL, Wan K, Zhou CH. Synthesis of novel sulfanilamide-derived 1,2,3-triazoles and their evaluation for antibacterial and antifungal activities. Eur J Med Chem. 2010;45:4631–4639. doi: 10.1016/j.ejmech.2010.07.031. [DOI] [PubMed] [Google Scholar]


