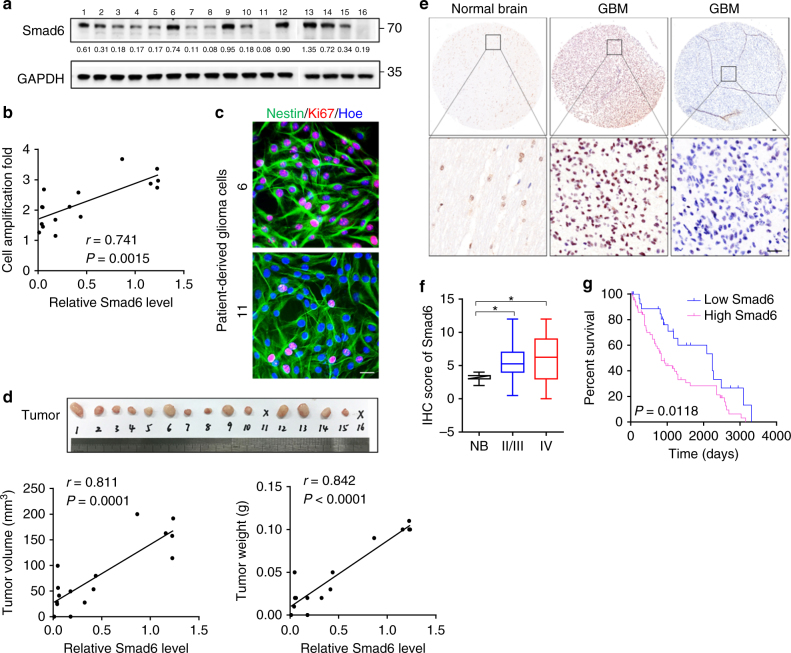
Fig. 1.
Smad6 is overexpressed in gliomas and predicts poor prognosis. a Smad6 expression in patient-derived glioma cells was examined by western blotting. GAPDH was used as a loading control. The relative quantification of Smad6 was listed. b Smad6 levels correlated to the proliferation capacity in patient-derived glioma cells (P- and r-values indicated, Spearman’s correlation analysis). Cells were cultured for 48 h and cell proliferation was monitored by a CCK-8 method. c Double immunofluorescence (IF) staining of Nestin and Ki67 in represented patient-derived glioma cells. Hoechst (Hoe) labeled the nuclei. Scale bars, 40 μm. d Smad6 expression correlated to tumor formation of patient-derived glioma cells (P- and r-values indicated, Spearman’s correlation analysis). Xenograft tumors derived from patient-derived glioma cells were shown in upper panel. The correlations of Smad6 to tumor volume and weight were shown in lower panel. e Immunohistochemistry (IHC) staining for Smad6 in glioma tissues. Scale bars, 50 μm. f Smad6 protein was frequently increased in nuclei of gliomas as compared it in normal brains (*P < 0.05, Unpaired t-test). g Overall survival analysis based on nuclear-Smad6 expression levels in glioma tissues. Groups were ranked according to nuclear-Smad6 IHC scores. The mean expression level of normal brains was used as a cutoff. The survival time of glioma patients with high Smad6 was significantly shorter than that of patients with low Smad6 (Log-rank χ2 = 6.348, P = 0.0118)