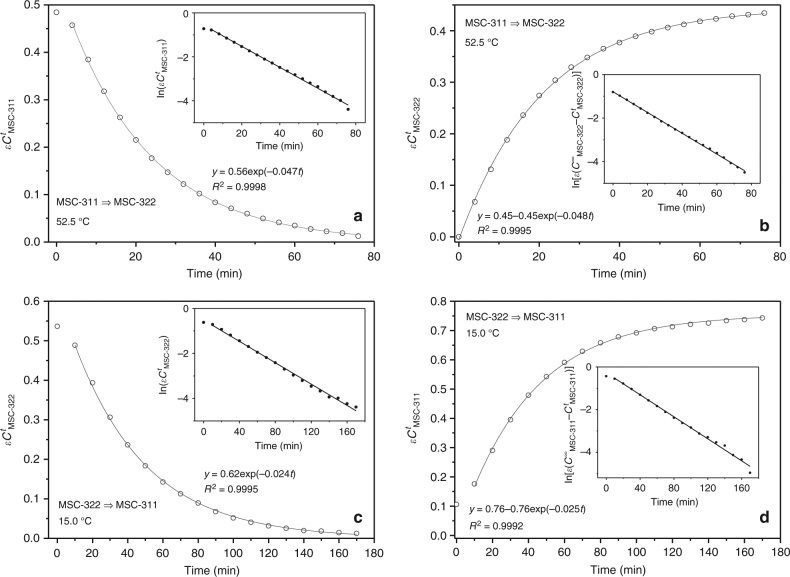
Fig. 4.
Kinetic study of the transformations at 52.5 and at 15.0 °C. The time-dependent concentrations (open circles) of the reactants (a, c) and products (b, d) for the MSC-311 to MSC-322 transformation at 52.5 °C (a, b) and the MSC-322 to MSC-311 transformation at 15.0 °C (c, d). Each of the two dispersions was prepared from one reaction mixture (~20 mg from the same approach used for Figs. 1 and 2) in toluene (3 mL). The rate constants obtained are 0.047 min−1 (a) and 0.024 min−1 (c), which match well those of 0.048 min−1 (b) and 0.025 min−1 (d), respectively