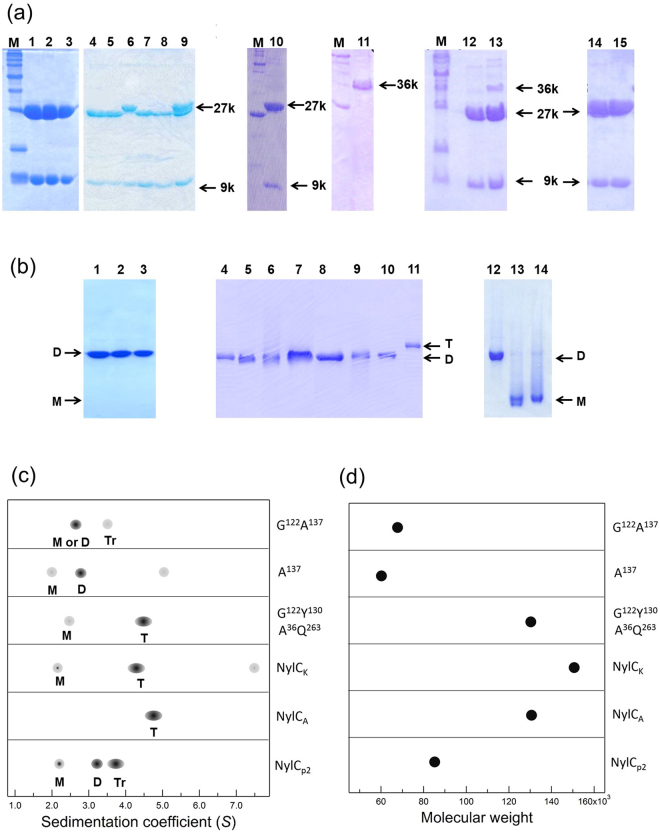
Figure 3.
Polyacrylamide gel electrophoresis and ultracentrifugation analysis of wild-type and mutant enzymes. (a) SDS-PAGE (17.5% gel) of purified NylC. Lane M, molecular size marker; Lane 1, NylCA; Lane 2, NylCK; Lane 3, NylCp2; Lane 4, NylCp2-K122; Lane 5, NylCp2-L122; Lane 6, NylCp2-R122; Lane 7, NylCp2-V122; Lane 8, NylCp2-Q122; Lane 9, NylCp2-N122; Lane 10, NylCp2-G122Y130A36Q263; Lane 11, NylCp2-A137; Lane 12, NylCp2-G122Y130; Lane 13, NylCp2-G122A137; Lane 14, NylCp2-G122; and Lane 15, NylCp2-Y130. (b) Native-PAGE (10% gel) of purified NylC. The wild-type and most NylC mutants exhibited single bands corresponding to the dimeric structure (shown as “D”). However, some mutants (NylCp2-A137 and NylCp2-G122A137) exhibited a faster-migrating band (presumed to be the monomer and shown as “M”). In contrast, the most thermostable mutant, G122Y130A36Q263, showed the slowest-migrating band (presumed to be tetrameric and shown as “T”). Lane 1, NylCA; Lane 2, NylCK; Lane 3, NylCp2; Lane 4, NylCp2-L122; Lane 5, NylCp2-N122; Lane 6, NylCp2-Q122; Lane 7, NylCp2-R122; Lane 8, NylCp2-K122; Lane 9, NylCp2-V122; Lane 10, NylCp2-G122; Lane 11, NylCp2-G122Y130A36Q263; Lane 12, NylCp2-G122Y130; Lane 13, NylCp2-A137; and Lane 14, NylCp2-G122A137. The grouping of portions cropped from different gels was made explicit using delineation with dividing white space. Full-length gels are included in a Supplementary Information file (Fig. S8). (c,d) Ultracentrifugation analysis. The subunit assembly of the wild-type enzymes (NylCp2, NylCA, and NylCK) and mutant enzymes derived from NylCp2 (G122Y130A36Q263, A137, and G122A137) was analyzed by sedimentation velocity (c) and sedimentation equilibrium methods (d). The estimated oligomeric states, which are the monomer (M), dimer (D), trimer (Tr), and tetramer (T), for each peak in (c) are marked. The deviations of the s values of the same oligomeric state suggest that the shape of the protein structure (spatial location of loop regions and bulkiness of protein) affects the sedimentation coefficient in aqueous solution. The wild-type NylCA gave a single peak (4.7 S) corresponding to a tetramer. The wild-type NylCK gave one major peak (4.3 S) and two minor peaks (7.5 S and 2.1 S), suggesting that the monomer, tetramer, and higher oligomers coexisted in equilibrium. The wild-type NylCp2 gave two major peaks [3.7 S (trimer) and 3.2 S (dimer)] and a minor peak (2.1 S) (monomer). The G122Y130A36Q263 mutant gave one major peak corresponding to 4.5 S (tetramer) and a minor peak (2.4 S) (monomer). The A137 mutant gave a major peak (2.7 S) (dimer) and two minor peaks [2.0 S (monomer) and 5.0 S] molecular species. The G122A137 mutant gave a major peak of 2.6 S (monomer or dimer) and a minor peak of 3.5 S (trimer).