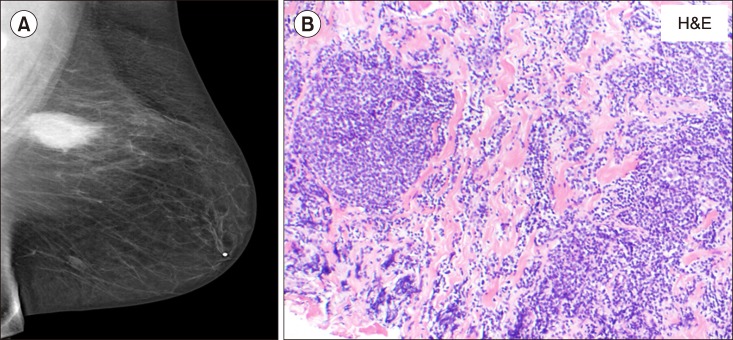
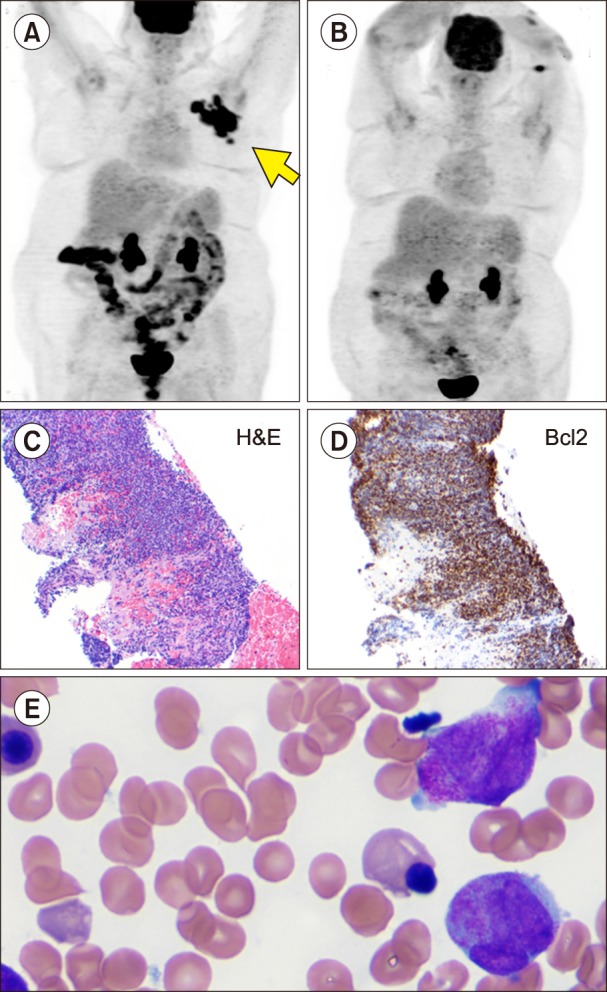
TO THE EDITOR: A 50-year-old Caucasian woman presented with gradually progressive fatigue, night sweats, and cellulitis of the lower abdominal wall in April 2000. In addition, she had morbid obesity, depression, osteoarthritis, and obstructive sleep apnea. Computerized tomography (CT) scan revealed lymphadenopathy above and below the diaphragm with moderately enlarged retroperitoneal and pelvic lymph nodes and hepatosplenomegaly. Laboratory data were unremarkable, with blood counts and serum lactate dehydrogenase levels within normal limits. CT-guided inguinal lymph node biopsy was consistent with follicular lymphoma (FL) [cluster of differentiation (CD)20 and Bcl2+]. Bone marrow biopsy revealed multifocal involvement with CD10-positive small lymphocytes distributed in the paratrabecular areas. She was diagnosed as having stage IVB FL. She was treated with 6 cycles of rituximab-cyclophosphamide, doxorubicin (Adriamycin), vincristine, and prednisone with 2 additional rituximab doses. She achieved complete remission (CR), as documented by CT scan and bone marrow examination. She then received maintenance interferon therapy for an additional 10 months and remained in CR for the next 4 years. In January 2005, a routine screening mammogram showed a 2.5-cm-sized mass in her left breast. Ultrasound-guided core needle biopsy was consistent with FL grade 2 (Fig. 1A, B). Immunohistochemistry analysis was positive for CD10, CD20, and BCL2 and negative for CD5. On positron emission tomography (PET)-CT scan, a focal area of hypermetabolism was noted in the left breast with standardized uptake value (SUV) of up to 5.4. There was no evidence of active lymphoma in any other area. Bone marrow biopsy was uninvolved with lymphoma and was suggestive of an extramedullary relapse of FL. She was treated under a clinical trial with 4 doses of rituximab 375 mg/m2 and bortezomib 1.6 mg/m2 weekly, every 25-day cycles. The patient had a partial response with a 25% reduction in the size of the breast mass; however, she also developed recurrence in the right-sided inguinal lymph nodes, as documented with PET-CT scans in the next 2 months. A fine needle aspirate confirmed the recurrence to be that of FL. A third salvage protocol consisting of rituximab and oblimersen, a bcl-2 inhibitor, was started in August 2005. She achieved CR with 1 cycle that lasted for 8 months. She again had an extramedullary relapse of FL in the left breast. She received a fourth salvage therapy with MG0103, an oral histone deacetylase inhibitor, with no response. She developed diffuse lymphadenopathy and splenomegaly that were hypermetabolic with a SUV up to 20 on a PET-CT scan. She received a fifth salvage therapy with 3 courses of rituximab, cyclophosphamide, etoposide, vincristine, and prednisone and had a transient partial response/stable disease; however, she progressed again with enlarged retroperitoneal nodes. In April 2007, a sixth salvage therapy with 4 courses of rituximab, mitoxantrone, ifosfamide, and etoposide (R-MINE) resulted in partial response. In July 2007, she again relapsed with bone marrow involvement; she was re-treated with 4 courses of R-MINE and achieved near CR until October 2008. A seventh salvage therapy with SB1518, an experimental JAK2 inhibitor, was started due to re-relapse. At this time, she also developed bilateral hydronephrosis due to retroperitoneal lymphadenopathy and underwent bilateral nephrostomy in October 2009. The drug was discontinued, and she was taken off the study. She was then treated with 4 courses of the eighth salvage therapy with rituximab and bendamustine until July 2010, with marked improvement of lymphadenopathy and decrease in spleen size, and she achieved CR, although with multiple infections and hematological toxicities. She had another extramedullary recurrence in April 2011 with PET-avid confluent lymphadenopathy (see arrow in Fig. 2A) in the left axilla (SUV, 12.4) biopsy showing FL grade 1. Fig. 2C and D shows a hematoxylin and eosin-stained section of the lymph node and immunostaining for Bcl2 was positive. In June 2011 (11 yr after the initial diagnosis of FL), she presented with pancytopenia and neutropenia, with white blood cell count of 2.3 K/UL, hemoglobin level of 9.1 gm/dL, platelet count of 14,000 K/UL, and 16% of blasts and promyelocytes. In addition, she developed a left-sided headache and was found to have a subdural hematoma on CT scan; her bone marrow evaluation revealed 42% of abnormal promyelocytes (Fig. 2E) and fluorescence in situ hybridization and polymerase chain reaction were positive for t (15;17) and promyelocytic leukemia protein-retinoic acid receptor alpha. A diagnosis of acute promyelocytic leukemia (APML) was made. The patient had early signs of cerebral herniation and was treated with decompression surgery with burr hole drainage for subdural hematoma. She was treated with 45 mg/m2 of oral all-trans retinoic acid (ATRA) and 0.15 mg/kg of intravenous arsenic trioxide (As2O3). One month after the induction therapy for APML, a remarkable decrease in the left axillary lymphadenopathy and improvement of metabolic activity were noted on a PET-CT scan (Fig. 2B). She received additional 4 courses of ATRA and As2O3 as a consolidation therapy and achieved complete cytogenetic and molecular remission of APML, which is maintained till date, i.e., 5 years after the completion of her therapy for APML. In the meantime, she was in CR for FL at 1.5 years after completion of ATRA with arsenic therapy. However, she developed an extramedullary relapse in her left upper arm, which is stable and is managed with observation.
Fig. 1. Mammogram showing extramedullary relapse of follicular lymphoma in the breast (A). Hematoxylin and eosinophil (H&E) staining of breast biopsy showing follicular lymphoma (B, ×100).
Fig. 2. Positron emission tomography scans showing response to all-trans retinoic acid (ATRA) with arsenic trioxide therapy: left axillary lymph node mass before (A) and after therapy (B), hematoxylin and eosinophil (H&E) staining of axillary lymph node showing follicular lymphoma (C, ×100), immunohistochemistry showing positive Bcl2 staining (D, ×100), bone marrow showing acute promyelocytic leukemia cells with multiple auer rods (E, ×1,000).
Our patient has a long history of relapsing and remitting course of FL for about 17 years. She had extramedullary relapses in the breast, axillary lymph node, and arm. She was heavily pretreated with multiple chemotherapies and biological therapies and was relatively refractory to most of the therapies currently used in the treatment of FL [1]. Our patient developed acute promyelocytic leukemia that was most likely secondary to the therapies that she received for FL. Although we expected her to achieve a remission of APL after ATRA and As2O3 therapy [2], we were surprised to observe that she also achieved a remission of FL following the ATRA and As2O3 therapy, which persisted for more than a year and stabilized her lymphoma. To our knowledge, only a small number of studies have reported on the therapeutic activity of As2O3 and ATRA in patients with FL, and none of these studies reported any responses that were as impressive as observed in our case. One phase II clinical trial of As2O3 for lymphoid malignancies showed that 0 of 3 patients with FL had a response to arsenic therapy in the frontline disease setting [3], whereas another phase II trial with 35 patients with relapsed refractory lymphoma (7 with FL) showed an overall response rate of 43%, with a median duration of response of 16 weeks [4]. There is modest efficacy of arsenic in myeloma, myelodysplastic syndrome, mantle cell lymphoma, and Burkitt's lymphoma [5,6,7]. One study reported that As2O3 can suppress mantle cell lymphoma cells by facilitating the polyubiquitination of cyclin D1 [8]. Another study [9] reported that KML001 (sodium meta-arsenite), an orally bioavailable arsenic compound, has significant anti-lymphoma activity. This agent induced G1 phase arrest via p27-induced inhibition of the kinase activities of cylin-dependent kinase (CDK)2, CDK4, and CDK6 and blocked cell signaling, including the signal transducer and activator of transcription, phosphatidylinositol-3 kinase/Akt, mitogen-activated protein kinase, and nuclear factor-κB signal pathways in KML001-treated Jurkat and Jurkat-R cells. The anti-lymphoma activity of KML001 was confirmed in a xenograft murine model. Furthermore, partial responses were observed in 1 patient with FL who was treated for 16 weeks without severe toxicities. In addition, a combination of oral As2O3 with chlorambucil and ascorbic acid was tested in patients with relapsed refractory mantle cell lymphoma (N=39). An overall response rate of 49% with a CR rate of 28% was achieved with this regimen, and only grade 1–2 toxicities were noted [7]. Similarly, ATRA therapy has also not shown any major benefit in lymphoma therapy, although in vitro evidence in 2 reports have suggested that B-cell lymphoma cell lines are sensitive to interferon gamma and ATRA [10], and mantle cell lymphoma cell can be inhibited by ATRA [11]. Precise mechanisms responsible for arsenic/ATRA combination activity in lymphoma have not been explored to date. It is therefore unclear whether this combination affects the differentiation pathway or facilitates the apoptosis or inhibits the cell proliferation in lymphoma cells. The data presented here, however, suggest that the combination of ATRA and As2O3 should be explored in preclinical studies and clinical studies in patients with relapsed refractory FL. The present case suggests that it is worthwhile to revisit the therapeutic activity of As2O3 and ATRA in lymphoid malignancies [12].
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Sehn LH, Fenske TS, Laport GG. Follicular lymphoma: prognostic factors, conventional therapies, and hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:S82–S91. doi: 10.1016/j.bbmt.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Mi JQ, Li JM, Shen ZX, Chen SJ, Chen Z. How to manage acute promyelocytic leukemia. Leukemia. 2012;26:1743–1751. doi: 10.1038/leu.2012.57. [DOI] [PubMed] [Google Scholar]
- 3.Chang JE, Voorhees PM, Kolesar JM, et al. Phase II study of arsenic trioxide and ascorbic acid for relapsed or refractory lymphoid malignancies: a Wisconsin Oncology Network study. Hematol Oncol. 2009;27:11–16. doi: 10.1002/hon.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao H, Sun G, Kong D, et al. A phase II study of arsenic trioxide in patients with relapsed or refractory malignant lymphoma. Med Oncol. 2015;32:79. doi: 10.1007/s12032-015-0526-x. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi S. Combination therapy with arsenic trioxide for hematological malignancies. Anticancer Agents Med Chem. 2010;10:504–510. doi: 10.2174/1871520611009060504. [DOI] [PubMed] [Google Scholar]
- 6.Berenson JR, Matous J, Swift RA, Mapes R, Morrison B, Yeh HS. A phase I/II study of arsenic trioxide/bortezomib/ascorbic acid combination therapy for the treatment of relapsed or refractory multiple myeloma. Clin Cancer Res. 2007;13:1762–1768. doi: 10.1158/1078-0432.CCR-06-1812. [DOI] [PubMed] [Google Scholar]
- 7.Gill H, Au WY, Cheung WW, Lee EY, Kwong YL. Oral arsenic trioxide-based regimen as salvage treatment for relapsed or refractory mantle cell lymphoma. Ann Oncol. 2014;25:1391–1397. doi: 10.1093/annonc/mdu142. [DOI] [PubMed] [Google Scholar]
- 8.Lo RK, Kwong YL. Arsenic trioxide suppressed mantle cell lymphoma by downregulation of cyclin D1. Ann Hematol. 2014;93:255–265. doi: 10.1007/s00277-013-1866-2. [DOI] [PubMed] [Google Scholar]
- 9.Yoon JS, Hwang DW, Kim ES, et al. Anti-tumoral effect of arsenic compound, sodium metaarsenite (KML001), in non-Hodgkin's lymphoma: an in vitro and in vivo study. Invest New Drugs. 2016;34:1–14. doi: 10.1007/s10637-015-0301-z. [DOI] [PubMed] [Google Scholar]
- 10.Niitsu N, Higashihara M, Honma Y. Human B-cell lymphoma cell lines are highly sensitive to apoptosis induced by all-trans retinoic acid and interferon-gamma. Leuk Res. 2002;26:745–755. doi: 10.1016/s0145-2126(01)00202-8. [DOI] [PubMed] [Google Scholar]
- 11.Singh AT, Evens AM, Anderson RJ, et al. All trans retinoic acid nanodisks enhance retinoic acid receptor mediated apoptosis and cell cycle arrest in mantle cell lymphoma. Br J Haematol. 2010;150:158–169. doi: 10.1111/j.1365-2141.2010.08209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barna G, Sebestyén A, Weischede S, et al. Different ways to induce apoptosis by fenretinide and all-trans-retinoic acid in human B lymphoma cells. Anticancer Res. 2005;25:4179–4185. [PubMed] [Google Scholar]