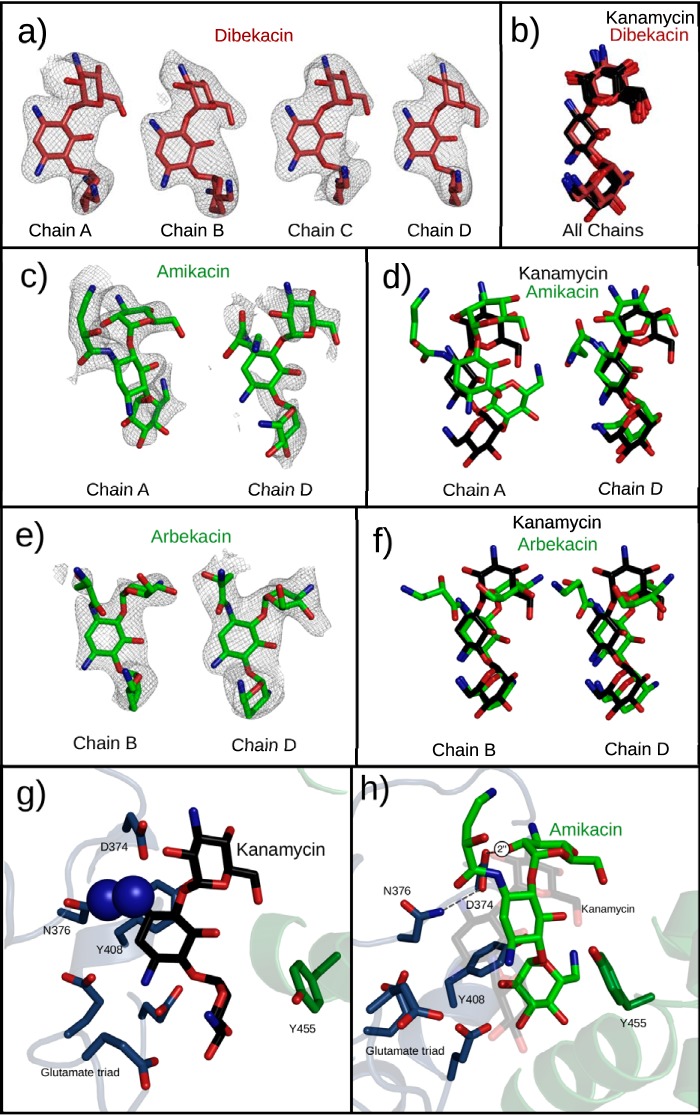
FIG 4.
Semisynthetic aminoglycosides observed in the active site of APH(2″)-Ia crystals. All structures are superimposed by alignment of the core regions of APH(2″)-Ia, not by using any atoms from the ligands. (a) Dibekacin binds with unambiguous electron density in all four chains of the APH(2″)-Ia crystal structure. Electron densities are Fo-Fc difference electron densities, contoured at 2.8 σ and carved 2.25 Å from the ligand. (b) Superpositioning of dibekacin and kanamycin molecules in respective structures generated by soaking of the aminoglycoside indicate a nearly perfect coincidence of the structures. (c) Amikacin shows a positive difference electron density in two chains of the APH(2″)-Ia structure upon cocrystallization. Electron densities are Fo-Fc difference electron densities, contoured at 2.0 σ and carved 1.75 Å from the ligand. (d) Superimposition with kanamycin indicates divergent (chain A) and shared (chain D) binding modes in the aminoglycoside binding pocket. (e) Arbekacin shows a positive difference electron density in two chains of the APH(2″)-Ia structure upon cocrystallization. Electron densities are Fo-Fc difference electron densities, contoured at 2.0 σ and carved 1.75 Å from the ligand. (f) Superimposition with kanamycin indicates that arbekacin shares the neamine-based interactions with APH(2″)-Ia but places the 4-linked aminohexose in a different conformation. (g) In the S376N mutant of APH(2″)-Ia, clashes between the antibiotic and the mutant residue prevent the binding of aminoglycosides by use of the neamine rings. (h) The S376N mutant can still support the binding of amikacin via its alternative binding conformation.