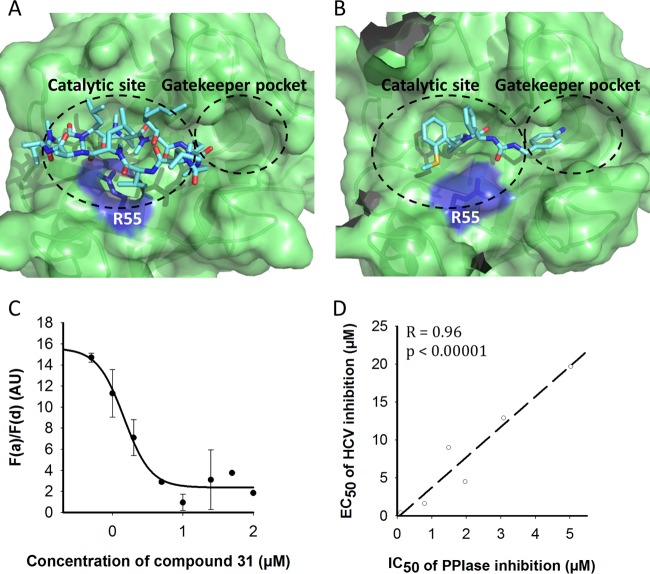
FIG 1.
Molecular modeling of the interaction of C31 and CsA with CypA, competition between C31 and CsA for CypA binding, and the relationship between the anti-PPIase activity of the SMCypIs in an enzyme assay and their anti-HCV activity against a genotype 1b HCV-SGR. (A and B) Surface representations of CypA in complex with CsA (A) and C31 (B) showing occupation of the catalytic site and the gatekeeper pocket. The side chain of Arg55 is represented in stick format and highlighted in purple. (C) Competition between C31 and CsA for CypA binding, assessed by a TR-FRET assay. The graphs represent the FRET emission ratios measured in the presence of increasing concentrations of C31. Unlabeled CsA and ALV were used as internal controls. The data are shown as means ± SD of results from three independent experiments. AU, arbitrary units. (D) Graph representing the relationship between the 50% inhibitory concentration (IC50) in a CypA PPIase enzyme assay and the EC50 in a genotype 1b HCV-SGR assay of 6 SMCypIs related to C31 (listed in Table S1 in the supplemental material). The Pearson correlation coefficient (R) and P value are shown on the graph.