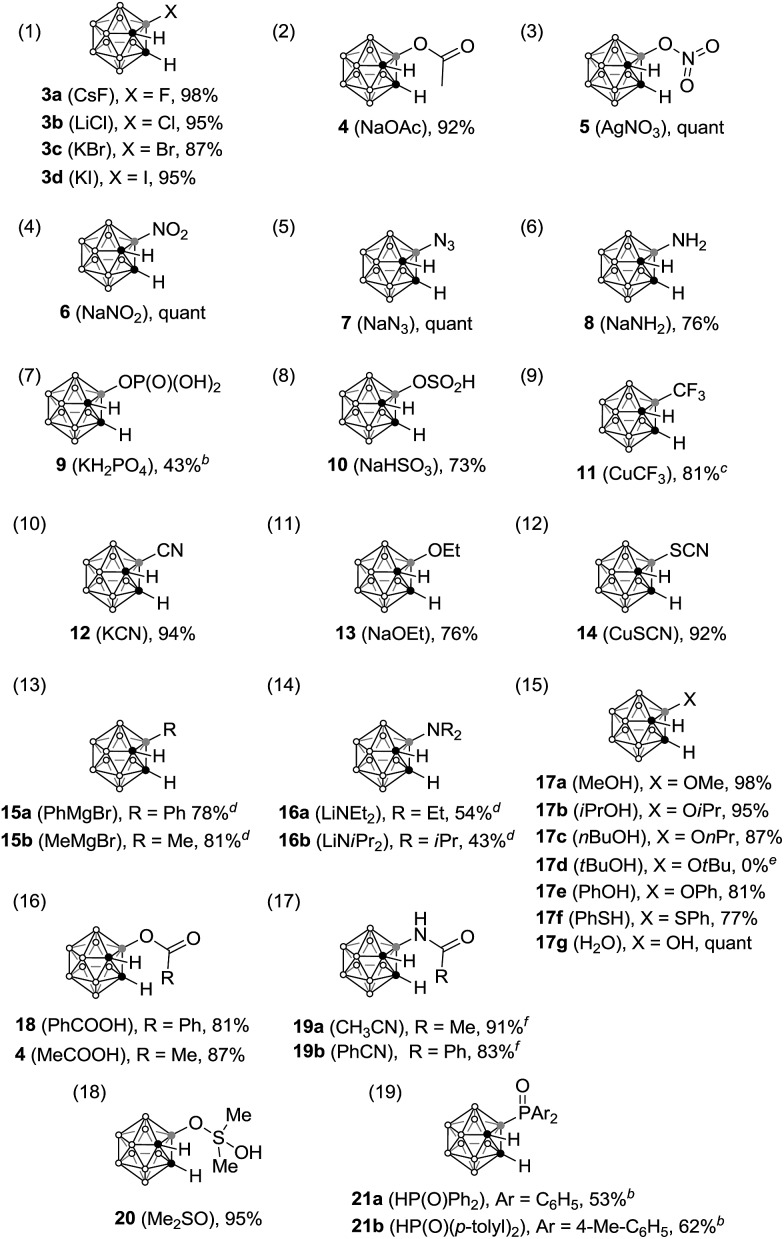
Table 1. Reaction of nucleophiles with precursor 1 a .
|
|
aReaction conditions: precursor 1 (0.1 mmol) was treated with nucleophile 2 (0.1 mmol for inorganic salt and phosphine oxide; 1.0 mmol for alcohol, acid and ketone; 0.4 mmol for Grignard reagent and lithium amide; nitriles were utilized as solvent) in CH3CN solution for 5 min; yields of isolated products are given.
bDeboronation occurred during purification.
cCuCF3 was prepared in situ from TMSCF3, CuSCN and Cs2CO3 in acetonitrile.16
d–78 °C, THF, 15 min.
eThe only product was 3-F-C2B10H11.
f50 °C, 6 h.
