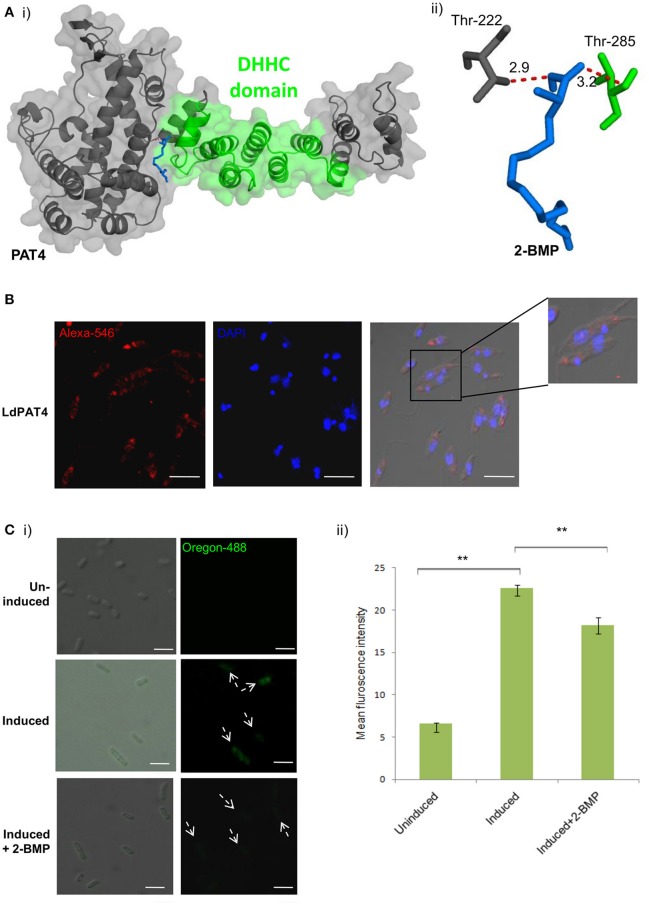
Figure 2.
(A) Docking study of LdPAT4 with palmitoylation inhibitor 2-BMP. (i) The protein accessible surface area was visualized for flexible ligand docking. (ii) Strong H-bonding indicated key residues involved in the interaction of the catalytic domain of LdPAT4 to 2-BMP. (B) Immunofluorescence analysis demonstrating expression and cellular localization of LdPAT4 in Leishmania promastigotes. Scale bar in white indicates 5 μM. (C) (i) Normaski and fluorescence images showing the catalytic activity of LdPAT4-DHHC using click chemistry in transformed E. coli cells. Scale bar in white indicates 2 μM. (ii) Fluorometer readings showing uninduced, induced and 2-BMP treated LdPAT4-DHHC expressing E. coli cells (**p < < 0.05). Data are represented as mean ± SEM.