Abstract
Background & objectives:
Significance of apoptosis as a prognostic marker is less well studied in paediatric acute lymphoblastic leukaemia (ALL) cases. Hence, a prospective study, involving 30 paediatric ALL cases, was done to assess the clinical relevance of in vivo apoptosis.
Methods:
Peripheral blood mononuclear cells from all patients were subjected to annexin V/propidium iodide staining to detect the degree of apoptosis [apoptotic index (AI)] at day 0 and day 35 post-induction chemotherapy. In addition, Bax and Bcl2 apoptotic protein expressions were studied at day 0 and their relative fluorescence mean intensity (RFMI) ratios were calculated.
Results:
Mean age of patients was 5.1 years. Of the 30 cases, 21 (70%) were at standard-risk, five (17%) at intermediate and four (13%) at high risk. Majority (83%) were B-ALL. Day 8 absolute blast count was >1000/μl in seven (23%) and <1000/μl in 23 of 30 (77%) cases. Day 35 marrow was M1 in 23 (92%) and M2 in two of 25 (8%) cases. AI at day 0 and day 35 ranged from 0.9 to16.6 per cent and 1.4 to 62.8 per cent with a mean of 5.90 and 19.64 per cent, respectively. The Bax/Bcl2 ratio ranged from 0.2 to 3.5 with a mean of 0.83. The ratio was predominantly anti-apoptotic, i.e. <1 (77%). A significant association was noted between low AI at day 0 and high total leucocyte count (P=0.02), T-cell phenotype (P=0.043) and high-risk as per NCI category (P=0.025). Significant increase (>30%) in day 35 AI was seen in only six cases.
Interpretation & conclusions:
Our study showed that low AI at day 0 was associated with a high-risk clinical phenotype in paediatric ALL. However, studies on larger group, especially with longer follow up or study of relapse cases, will help draw conclusions regarding apoptosis assessment in paediatric ALL.
Keywords: Acute lymphoblastic leukaemia, apoptotic index, Bax/Bcl2 ratio, paediatric, ratio, spontaneous apoptosis, treatment
Acute lymphoblastic leukaemia (ALL) is the most common malignancy in children. It accounts for 25 per cent of all childhood cancers and approximately 75 per cent of all cases of childhood leukaemias1. Progress in the treatment of acute leukaemia has been achieved by the development of empirically designed chemotherapy protocols applying different cytotoxic drugs in an optimized time course, and at present, a cure rate of 80-85 per cent can be achieved1.
Despite the rampant use of genomics and microarray techniques to discover new prognostic markers in paediatric ALL, factors such as age, initial total leucocyte count (TLC) at presentation and early response to chemotherapy as measured on day 7/14 and minimal residual disease (MRD) post induction therapy, still remain the single most important independent prognostic factors influencing the final treatment outcome2. However, recent interest on chemotherapy drug resistance has renewed the focus on the role of apoptosis pathways in leukaemia treatment and outcome, and there is an emerging realization that cancer chemotherapeutic agents act primarily by inducing cancer cell death through the mechanisms of apoptosis. In vitro studies with tumour and leukaemia cell lines have shown that cytotoxic drugs used in anticancer chemotherapy induce cell death by the activation of diverse apoptosis signalling pathways3,4,5,6,7,8. Based on the results of these in vitro studies, it has been suggested that functional defects in apoptosis signalling molecules or deficient activation of apoptosis pathways are responsible for chemotherapy resistance and treatment failure in acute leukaemia. The involvement of apoptosis in anticancer chemotherapy is also supported by studies demonstrating changes of death receptor gene expression levels in primary leukaemia cells upon drug treatment in vitro9,10,11.
However, only a few studies have analyzed apoptosis in leukaemia cells during anti-leukaemic treatment in vivo. The detection of apoptotic leukaemia cells in the peripheral blood during chemotherapy has primarily failed, possibly because of the rapid removal of pro-apoptotic cells from the circulation. Banker et al3 showed variable degree of spontaneous apoptosis in de novo acute myeloid leukaemia (AML) samples and showed less treatment-related apoptosis, thereby suggesting that apoptotic responses to therapeutic agents may be frequently attenuated in AML cases. A study by Ong et al12 found high Bax levels to be associated with a favourable prognosis in AML patients.
There is still controversy regarding the significance of apoptosis during remission induction therapy, and there are very few studies regarding this topic in paediatric ALL, especially in our setting. Moreover, there remains a lacuna on the usefulness of apoptotic index (AI) and apoptotic protein ratio measurement as a prognostic marker in paediatric ALL. Hence, this study was undertaken to evaluate the prognostic relevance of in vivo apoptosis in paediatric ALL cases by noting the association between degree of pre- and post-treatment apoptosis and expression ratio of apoptotic proteins with early treatment response parameters.
Material & Methods
A prospective study in which 30 consecutive paediatric ALL cases admitted for the treatment in Haematology-Oncology Unit of department of Paediatrics, Postgraduate Institute of Medical Education & Research, Chandigarh, India, over a period of one year (July 2014-June 2015) were enrolled. The cases were confirmed as ALL on bone marrow examination and flow cytometry-based immunophenotyping using standard panel of monoclonal antibodies. The study was duly approved by the ethics committee of the institute. Written informed consent was obtained from parents/guardians. Peripheral blood EDTA samples (2-3 ml) were collected before start of chemotherapy. The samples for assessing degree of apoptosis were collected and processed twice: at presentation (day 0-diagnosis) and at day 35 (check marrow-post-induction) (Fig. 1). The samples collected were processed within one hour of collection to minimize further spontaneous apoptosis ex vivo.
Fig. 1.
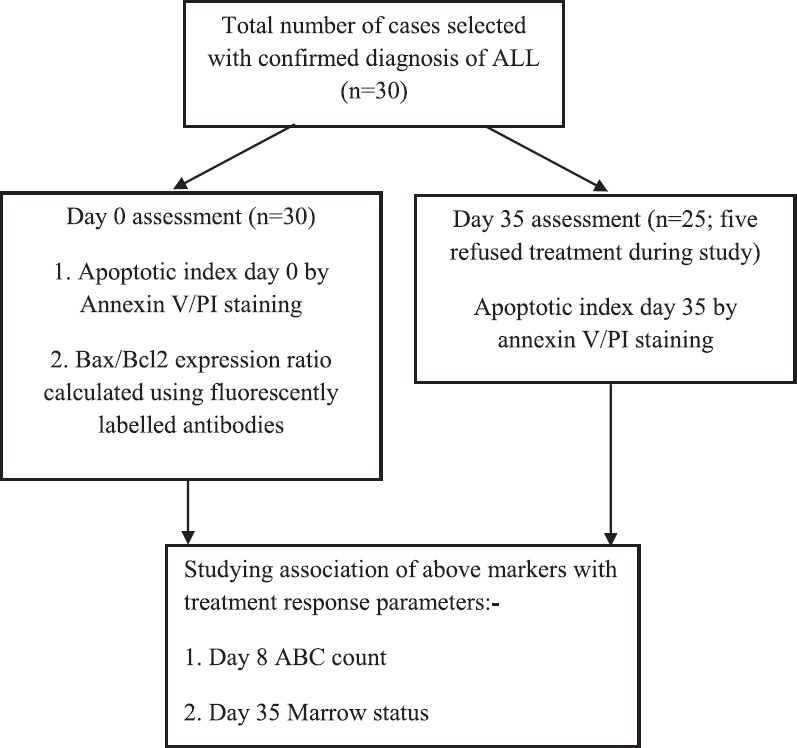
Flowchart showing the study design. ABC, absolute blast count; PI, propidium iodide; ALL, acute lymphoblastic leukaemia.
Inclusion criteria included enrolment of all consecutively diagnosed paediatric ALL cases during the study period with age range of 0-12 yr and obtaining consent for treatment at our institute. Cases of paediatric AML or biphenotypic acute leukaemia, old follow up cases of ALL on chemotherapy and those who refused consent for enrolment were excluded from the study.
Flow cytometry methodology for apoptosis assessment: Percentage/degree of cells showing apoptosis as detected by the annexin V and propidium iodide (PI) staining procedure was carried out twice: once at presentation before the start of chemotherapy (day 0-termed spontaneous apoptosis) and then at day 35 check marrow following early induction chemotherapy (day 35- termed post-treatment apoptosis). Peripheral blood mononuclear cells (PBMC) were prepared and purified using density-gradient centrifugation (using Ficoll-Hypaque medium, GE Healthcare, USA). To 2-3 ml blood in a conical tube, equal amounts of phosphate-buffered saline (PBS, pH 7.4) was added followed by 2.0 ml of Ficoll-Hypaque layered at the bottom of the tube. Centrifugation was done for 30 min at 500 g at room temperature (RT). The mononuclear cell layer at interface was aspirated into a separate tube. The cells were then counted under haemocytometer (Hausser Scientific Bright-Line, USA) to ensure the presence of at least 1.0×106/μl cells. The staining procedure was performed using fluorescein isothiocyanate (FITC) annexin V apoptosis detection kit (BD Biosciences; Catalogue No. 556570, USA) for the use on BD FACS flow cytometer (LSR-II). The cells were washed twice with cold PBS and then re-suspended in binding buffer at a concentration of 1×106 cells/ml; 100 μl of the cell solution was then transferred to a 5 ml culture tube. This was followed by addition of 5 μl of FITC annexin V and 5 μl propidium iodide (PI) in the cell-binding buffer suspension. Cells were gently vortexed and incubated for 15 min at RT (25°C) in the dark. Finally, 400 μl of binding buffer was added to each tube and the samples were analyzed by flow cytometry within one hour.
The following four controls were used to set up compensation and quadrants for optimal interpretation of apoptosis results: Tube 1-unstained cells, tube 2-cells stained with FITC annexin V only (no PI), tube 3-cells stained with PI only (no FITC annexin V) and tube 4-containing both annexin V and PI.
The percentage of cells positive for apoptosis was graded as follows:
Cells positive for annexin V only and PI negative: Early apoptotic cells;
Cells positive for both annexin V and PI: Late apoptotic cells and necrotic cells;
Cells negative for both dyes: Viable/live cells.
Although late apoptosis was noted as both annexin V and PI positive, the same could also include necrotic cells and was not truly representative of spontaneous or drug-induced apoptosis. Hence, only cells positive for annexin V and negative for PI (early apoptosis) were taken as true apoptotic cells. The AI was calculated for each sample run at both time intervals (spontaneous and post-treatment) as follows13:
AI per cent=Number of apoptotic cells/total number of cells examined×100
The overall AI was expressed as mean±standard deviation (SD) and compared between pre- and post-treatment time intervals. The respective mean values were taken as cut-offs for defining high and low apoptosis groups at both time intervals. In addition, a difference of >30 per cent in AI value post-chemotherapy as compared to pre-treatment value for each sample was taken as a significant difference accounting for increase in apoptosis following chemotherapy13.
Staining procedure for Bax and Bcl2 apoptotic proteins: This was performed only once on the peripheral blood cells in a case of ALL at day 0. The mononuclear cells separated were fixed with 1 ml ice-cold 90-100 per cent cold methanol for 30 min at 4°C in dark. This was followed by washing with PBS at 500 g for five minutes and the supernatant was discarded. The permeabilization buffer (containing 0.15% Triton X-100 and 1% BSA in TBS) was then added to the fixed cells and incubated for 10 min at RT. The cells were aliquoted to two different conical tubes; 20μl of FITC-labelled Bcl2 and phycoerythrin (PE)-labelled Bax antibodies were put into the two separately labelled tubes, respectively, and incubated in dark for 30 min. Washing with PBS was done, followed by resuspension of cells in adequate amount of buffer solution to run on flow cytometer within 1-2 h. Before the first run, isotype negative controls for FITC and PE were run to note compensation values for both fluorescent dyes. Both the proteins showed cytoplasmic and perinuclear positivity and the percentage of blast cells (high FSC vs SSC) positive in each case were noted and the Bax/Bcl2 ratio was calculated as follows:
Relative mean fluorescence Bcl2=mean fluorescence Bcl2−mean fluorescence isotype control (FITC)
Relative mean fluorescence Bax=mean fluorescence Bax−mean fluorescence isotype control (PE)
Bax/Bcl2 ratio=relative mean fluorescence intensity (RMFI) of Bax/RMFI of Bcl2
Bax/Bcl2 ratio was classified as anti-apoptotic ratio if <1 and pro-apoptotic ratio if >1. All ALL cases were treated as per Indian Childhood Collaborative Leukemia study group (ICiCLe) protocol. The protocol is a collaborative multicentric childhood leukemia treatment trial initiated in 2014 and as per the trial all children with acute lymphoblastic leukemia are being uniformly treated by important collaborating centres all over India. It is an Indian risk adapted and stratified treatment protocol based on UK MRC 2003 protocol version 7 and Berlin Frankfurt Munster (BFM) protocol14. The protocol has a seven days prednisolone pre-phase followed by 3 or 4 drug induction regimen based on NCI, cytogenetic and molecular data risk stratification. Post induction MRD is assessed at day 35 and further consolidation regimen is risk stratified based on MRD status. The cases at presentation were classified as per National Cancer Institute (NCI) risk criteria15 into standard-, intermediate- and high-risk groups according to age, immunophenotype and TLC count. Day 8 absolute blast count (ABC) after seven days of prednisolone decided the further 3- or 4-drug intensified induction regimen (vincristine, prednisolone and L-asparaginase in standard risk and plus daunorubicin if intermediate-2 doses/high risk-4 doses). Intrathecal methotrexate was also given in each case as per the protocol.
For all cases, demographic, clinical presentation, haematological presentation and immunophenotype data were collected. In addition, RT-PCR for recurrent translocations (t12;21, t1;19, t9;22 and t4;11) are routinely performed in all new cases of ALL enrolled for treatment and data related to same was also noted16. Early response to chemotherapy parameters were defined as per the ICiCLe protocol:
Day 8: good prednisolone responders (GPPs): If ABC at day 8 < 1000/μl and poor prednisolone responders (PPRs) if ABC at day 8 ≥ 1000/μl.
Day 35 bone marrow remission criteria: M1 status (<5% blasts), M2 (5-20% blasts) and M3 status (>21% blasts).
Statistical analysis: Data were analyzed using SPSS software version 22 (IBM Corp., USA). The data were categorized into different categories. The baseline variables in various groups were shown as mean±SD for quantitative variables and as percentages for qualitative variables. Univariate analysis was done to see the association between various groups using Chi-square test or Fisher's exact tests whichever was applicable. Parametric tests were done.
Results
Peripheral blood samples from 30 children with ALL were collected and processed at day of diagnosis (day 0) and post-induction therapy day (day 35) to note for the degree of apoptosis. In addition, expression of anti-(Bcl2) and pro-apoptotic (Bax) proteins was studied at diagnosis. There were 22 (73%) male and eight (27%) female cases, with a male:female ratio of 2.75:1. The mean age was 5.1±3.4 yr with age range 1.2-11 years. There were 27 (90%) with age between 1 and 10 yr and only three (10%) with age <1 or >10 yr. All cases had varying degrees of hepatomegaly and splenomegaly at presentation. However, marked hepatomegaly (>7 cm below right costal margin) and marked splenomegaly (>10 cm below left costal margin) were noted in three (10%) cases each, respectively. Based on TLC, age and immunophenotype at diagnosis, 21 of 30 (70%) were in standard-risk, five (17%) intermediate-risk and four (13%) in high-risk category. None of the cases had cerebrospinal fluid positivity or central nervous system disease at diagnosis. Twenty four (80%) patients had TLC less than 50×109/l at presentation while only six (20%) had TLC > 50×109/l. Only five (17%) were T-ALL and rest 25 (83%) were B-ALL. Recurrent translocations by RT-PCR were noted in four (13.0%). Day-8 absolute blast count in the peripheral blood to check for prednisolone response was >1000/μl in seven (23%; poor responders) and <1000/μl in 23 (77%; good responders) cases. Of the 30 cases, five refused further treatment after day 8-15 of diagnosis, and hence, assessment for apoptosis post-induction could not be checked in these cases. Post-induction, day-35 check marrow status was M1 in 23 (92%) and M2 in two of 25 (8%) cases. None of the cases had >25 per cent (M3) blasts in bone marrow on day 35. Day 35 MRD as assessed by flow cytometry with a sensitivity of 0.01 per cent was available in all B-ALL cases but not in four T-ALL cases. Twenty one of the 25 cases in whom MRD results were available; five (24%) had a high MRD of >0.01 per cent. Subsequently, these patients were upgraded to high-risk disease, and consolidation treatment was intensified with high doses of methotrexate. All 25 cases were alive at the last follow up with a median follow up duration of three months (range 2-8 months).
Spontaneous and post-treatment apoptosis: Spontaneous AI at day 0, as assessed by annexin V-positive and PI-negative cells, ranged from 0.9 to 16.6 per cent with a mean±SD of 5.90±4.5 per cent and a median of 4.50 per cent (Table I). The post-induction treatment apoptosis (AI at day 35) as assessed on day 35 ranged from 1.4 to 62.8 per cent with a mean ± SD of 19.64±17.39 per cent and a median of 14.0 per cent. Although the mean difference in pre- and post-treatment AI was high, a significant increase in apoptosis between two time intervals as defined by a cut-off of 30 per cent was noted in only six of 25 (24%) cases. The day-35 AI and day-0 AI of a few samples are shown in Fig. 2A and B, respectively.
Table I.
Apoptosis index (AI) and apoptotic protein expression data (n=30 samples-apoptotic index day 0 and n=25 apoptotic index day 35)
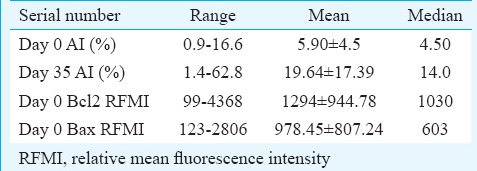
Fig. 2.
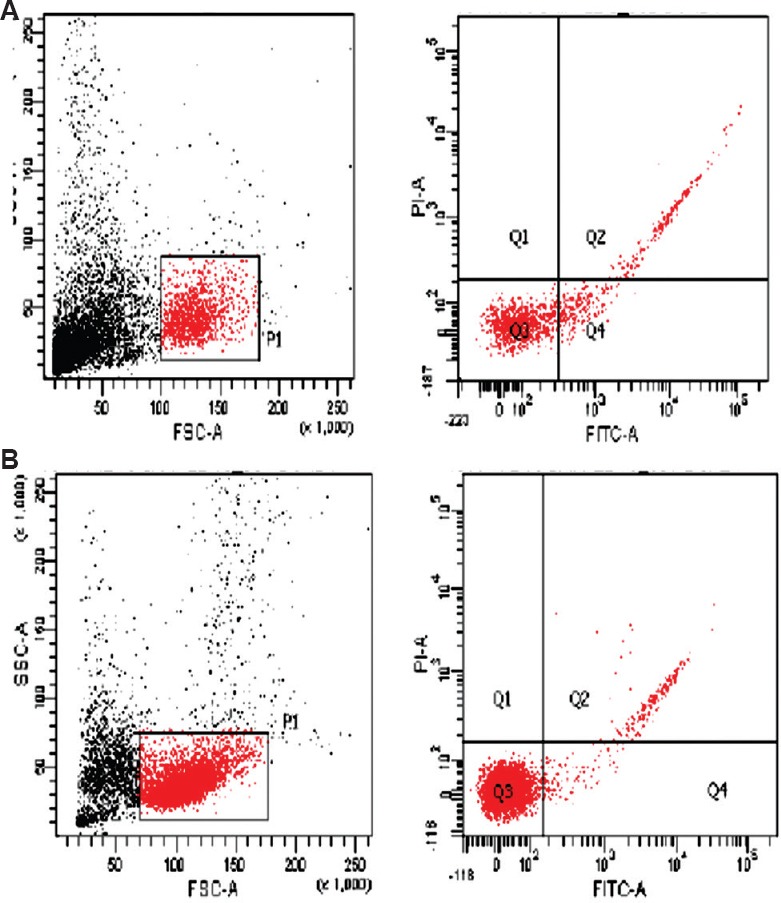
(A) High day 35 apoptosis index in a case (quadrant Q4-16.9%); (B) low day 0 apoptosis index in a case (quadrant Q4-1.6%).
The anti-apoptotic and pro-apoptotic proteins assessed in the study were Bcl2 and Bax, respectively. Their cytoplasmic expression was studied using fluorescent-labelled markers by flow cytometry at day of diagnosis of ALL (day 0). The RMFI for Bcl2 ranged from 99 to 4368 with cellular positivity ranging from 78.1 to 100 per cent. The RFMI for Bax ranged from 123 to 2806 with cellular positivity ranging from 73.2 to 100 per cent. The Bax/Bcl2 ratio ranged from 0.2 to 3.5 with a mean of 0.83. The ratio was anti-apoptotic, i.e. <1 in 23 of 30 (77%) cases, while pro-apoptotic ratio, i.e.>1, in only seven of 30 (23%) cases (Table II). The flow positivity of Bax and Bcl2 proteins is shown in Fig. 3A and B, respectively.
Table II.
Correlation of demographic, clinico-haematological and chemotherapy response variables with apoptotic index and apoptotic protein ratio
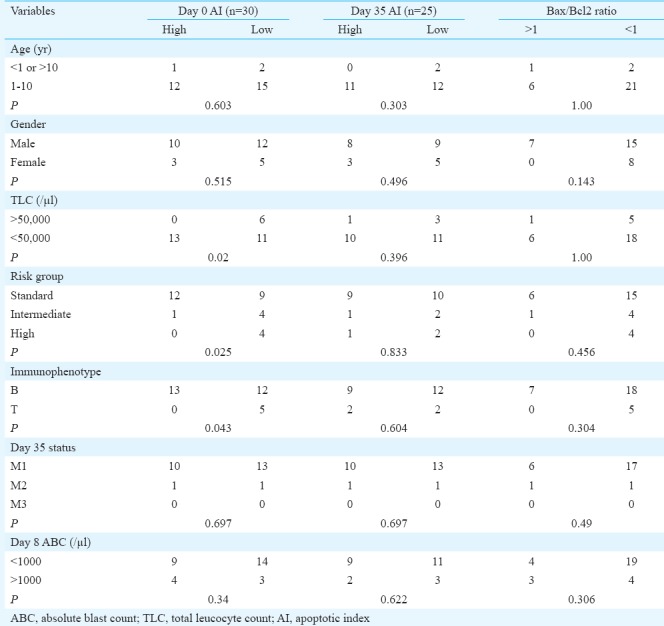
Fig. 3.
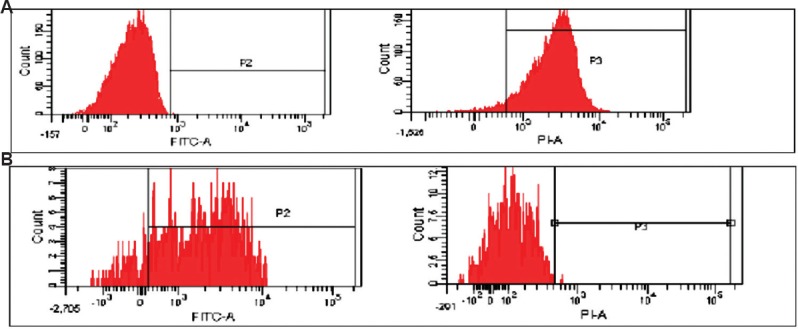
(A) High Bax mean fluorescence intensity in a case, PE (phycoerythrin) labelled; (B) high Bcl2 mean fluorescence intensity in a case, FITC (fluorescein isothiocyanate) labelled.
Association of pre- and post-treatment apoptosis index (AI) and apoptotic protein ratio with demographic, clinico-haematological variables and response to chemotherapy:Chi-square test was performed to correlate AI pre- and post-treatment and apoptotic protein ratio with various clinical, haematological and treatment response parameters (Table II). A significant association of low spontaneous apoptosis was noted with TLC at diagnosis, risk category and immunophenotype. All (100%) cases with high TLC (>50,000/μl) had a low spontaneous apoptosis (day 0) as compared to only 11 of 24 (46%) cases with low TLC count (P=0.011). None of the cases with high spontaneous AI belonged to high-risk category compared to 30 per cent of those with low AI who were high risk at diagnosis (P=0.026). In addition, all five T-ALL cases (100%) had low spontaneous AI as compared to 12/25 (48%) B-ALL cases (P=0.043). Post-induction treatment-related AI (day 35) had no association with any of the clinical or treatment response-related parameters (Table II).
A pro-apoptotic Bax/Bcl2 ratio (<1) was significantly associated with massive hepatomegaly (P=0.008). No association was noted between AI day 0 or day 35 with Bax/Bcl2 ratio.
Discussion
Degree or percentage of spontaneous apoptosis in acute leukaemia cases at diagnosis can be measured by a variety of methods. These include culturing of the cells in vitro followed by staining with fluorescent dyes such as acridine orange and evaluating apoptosis under fluorescent microscope or using flow cytometry to note the percentage of apoptotic cells by staining with DNA dyes such as PI, noting the loss of mitochondrial potential using JC1 dye (tetraethylbenzimidazolylcarbocyanine iodide)17, the Tunnel technique to note chromatin condensation or by targeting the cell surface phosphatidyl serine residues expressed by apoptotic cells using the FITC-labelled annexinV marker18. In a study by Savitskiy et al19, a comparative analysis of all five different methods used to evaluate apoptosis, as highlighted above, was done. It was found that ALL cells were significantly more sensitive to spontaneous apoptosis detection using Annexin V staining versus AML cells. The least sensitive technique was apoptosis detection by sub-G1-peak/PI-staining, and in patients with B-lineage ALL, strong positive correlation existed between the level of cells with loss of mitochondrial membrane potential (JC1), chromatin condensation (AO) and externalization of phosphatidylserine (annexin V+PI+). In the present study, in vivo detection of apoptosis was done pre and post-treatment using annexin V staining method. Measurable apoptosis was found at diagnosis as well as post-treatment from peripheral blood, implying that flow cytometry was sensitive technique to pick low number of apoptotic cells as well, provided the samples were processed and run rapidly without much delay. The mean spontaneous AI at day 0 in our study was 5.90±4.5 per cent and at day 35 post-treatment was 19.64±17.39 per cent. Although there were six cases with more than 30 per cent increase in AI post-treatment, the degree of apoptosis in rest of the cases post-treatment was either comparable or marginally more than spontaneous AI. Malinowska et al13 studied spontaneous and post-initial treatment apoptosis index and blast pH in 38 children with acute leukaemia and found the mean spontaneous AI 19±16 per cent and post-early treatment was 17±11 per cent. They concluded that this could indicate that the amount of spontaneous apoptosis, which is a measure of intrinsic cellular sensitivity to apoptosis, was distinct from apoptosis caused by cytotoxic agents. Stahnke et al20 demonstrated significant increase in apoptosis in CD34+ leukaemic cells ex-vivo after culture for 48 h in a cytotoxic drug medium. We assessed apoptosis post-treatment at day 35, and it is possible that due to rapid clearance of apoptotic bodies from peripheral blood, we were not able to have a significant increase in apoptosis in most samples.
A significant association was noted between low AI at day 0 and high TLC (P=0.02), T-cell phenotype (P=0.043) and high-risk NCI category (P=0.025). Malinowska et al13 found all unfavourable events (primary resistance to treatment, relapse and death) to be associated with patients with spontaneous AI below the median; however, we did not find any relation of low AI at diagnosis with treatment response parameters. Hence, it is difficult to comment upon prognostic value of low AI at diagnosis on the basis of high-risk phenotype.
Day 35 AI in our study did not have any association with standard risk factors studied. Moreover, no significant difference in day 0 and day 35 AI between responders and non-responders was noted in our study. Bushan et al5 studied the relation of cell viability and apoptosis at diagnosis in ALL cases with clinical outcome to induction therapy using the flow cytometry method. They found that patients with ALL who achieved complete remission (CR) had significantly lower mean live cell (70.9%) compared to those patients who did not achieve CR (93.3%). Furthermore, in contrast to the results of our study, their study highlighted that ALL responders also had significantly higher mean early apoptotic cell (19.4%) as compared to non-responders (5%).
The Bax/Bcl2 ratio in our study ranged from 0.2 to 3.5 with a mean of 0.83. The ratio was predominantly anti-apoptotic, i.e. <1 in 77 per cent cases, while pro-apoptotic ratio, i.e. >1, in only 23 per cent cases. Aref et al1 reported that Bcl2 expression at diagnosis in ALL was related to responsiveness to induction therapy but not with patient outcome. Coustan-Smith et al6 found no relation between Bcl2 levels and disease aggressiveness or resistance to therapy. Hogarth and Hall21 also found that high expression of Bax at diagnosis in ALL was associated with high risk of relapse, while Prokop et al22 suggested that Bax/Bcl2 ratio rather than expression of Bax or Bcl2 alone has more prognostic value in ALL. Kaparou et al23 found high Bax/Bcl2 ratio at diagnosis to be associated with high-risk patient profile in ALL. However, our study failed to show any prognostic importance of Bax/Bcl2 ratio in paediatric ALL.
In conclusion, definitive comments on prognostic importance of AI and apoptotic protein ratio in paediatric ALL cases could not be made from the present study as the sample size was less and follow up data were short and limited; the study highlighted that low spontaneous AI at day 0 was associated with a high-risk patient phenotype.
Footnotes
Financial support & sponsorship: None
Conflicts of Interest: None.
References
- 1.Aref S, Salama O, Al-Tonbary Y, Mansour A. Assessment of Bcl-2 expression as modulator of fas mediated apoptosis in acute leukemia. Hematology. 2004;9:113–21. doi: 10.1080/1024533042000205496. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: An update. J Clin Oncol. 2011;29:551–65. doi: 10.1200/JCO.2010.30.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banker DE, Groudine M, Norwood T, Appelbaum FR. Measurement of spontaneous and therapeutic agent-induced apoptosis with BCL-2 protein expression in acute myeloid leukemia. Blood. 1997;89:243–55. [PubMed] [Google Scholar]
- 4.Belaud-Rotureau MA, Durrieu F, Labroille G, Lacombe F, Fitoussi O, Agape P, et al. Study of apoptosis-related responses of leukemic blast cells to in vitro anthracycline treatment. Leukemia. 2000;14:1266–75. doi: 10.1038/sj.leu.2401803. [DOI] [PubMed] [Google Scholar]
- 5.Bhushan B, Ahuja D, Verma S, Saluja S, Siddiqui S, Kapur S, et al. Relation of cell viability and apoptosis with clinical remission following induction chemotherapy in ALL and AML. J Exp Clin Cancer Res. 2007;26:313–21. [PubMed] [Google Scholar]
- 6.Coustan-Smith E, Kitanaka A, Pui CH, McNinch L, Evans WE, Raimondi SC, et al. Clinical relevance of BCL-2 overexpression in childhood acute lymphoblastic leukemia. Blood. 1996;87:1140–6. [PubMed] [Google Scholar]
- 7.Decaudin D, Geley S, Hirsch T, Castedo M, Marchetti P, Macho A, et al. Bcl-2 and Bcl-XL antagonize the mitochondrial dysfunction preceding nuclear apoptosis induced by chemotherapeutic agents. Cancer Res. 1997;57:62–7. [PubMed] [Google Scholar]
- 8.Dive C, Evans CA, Whetton AD. Induction of apoptosis - New targets for cancer chemotherapy. Semin Cancer Biol. 1992;3:417–27. [PubMed] [Google Scholar]
- 9.Friesen C, Herr I, Krammer PH, Debatin KM. Involvement of the CD95 (APO-1/FAS) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nat Med. 1996;2:574–7. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- 10.Hannun YA. Apoptosis and the dilemma of cancer chemotherapy. Blood. 1997;89:1845–53. [PubMed] [Google Scholar]
- 11.Wen J, Ramadevi N, Nguyen D, Perkins C, Worthington E, Bhalla K, et al. Antileukemic drugs increase death receptor 5 levels and enhance Apo-2L-induced apoptosis of human acute leukemia cells. Blood. 2000;96:3900–6. [PubMed] [Google Scholar]
- 12.Ong YL, McMullin MF, Bailie KE, Lappin TR, Jones FG, Irvine AE, et al. High bax expression is a good prognostic indicator in acute myeloid leukaemia. Br J Haematol. 2000;111:182–9. doi: 10.1046/j.1365-2141.2000.02315.x. [DOI] [PubMed] [Google Scholar]
- 13.Malinowska I, Stelmaszczyk-Emmel A, Wasik M, Rokicka-Milewska R. Apoptosis and pH of blasts in acute childhood leukemia. Med Sci Monit. 2002;8:CR441–7. [PubMed] [Google Scholar]
- 14.Chang JE, Medlin SC, Kahl BS, Longo WL, Williams EC, Lionberger J, et al. Augmented and standard Berlin-Frankfurt-Munster chemotherapy for treatment of adult acute lymphoblastic leukemia. Leuk Lymphoma. 2008;49:2298–307. doi: 10.1080/10428190802517732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith M, Arthur D, Camitta B, Carroll AJ, Crist W, Gaynon P, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 16.Bhatia P, Binota J, Varma N, Bansal D, Trehan A, Marwaha RK, et al. Incidence of common chimeric fusion transcripts in B-cell Acute Lymphoblastic Leukemia (ALL): An Indian perspective. Acta Haematol. 2012;128:17–9. doi: 10.1159/000338260. [DOI] [PubMed] [Google Scholar]
- 17.Williams O. Flow cytometry-based methods for apoptosis detection in lymphoid cells. Methods Mol Biol. 2004;282:31–42. doi: 10.1385/1-59259-812-9:031. [DOI] [PubMed] [Google Scholar]
- 18.Los M, Herr I, Friesen C, Fulda S, Schulze-Osthoff K, Debatin KM, et al. Cross-resistance of CD95- and drug-induced apoptosis as a consequence of deficient activation of caspases (ICE/Ced-3 proteases) Blood. 1997;90:3118–29. [PubMed] [Google Scholar]
- 19.Savitskiy VP, Shman TV, Potapnev MP. Comparative measurement of spontaneous apoptosis in pediatric acute leukemia by different techniques. Cytometry B Clin Cytom. 2003;56:16–22. doi: 10.1002/cyto.b.10056. [DOI] [PubMed] [Google Scholar]
- 20.Stahnke K, Eckhoff S, Mohr A, Meyer LH, Debatin KM. Apoptosis induction in peripheral leukemia cells by remission induction treatment in vivo: Selective depletion and apoptosis in a CD34+ subpopulation of leukemia cells. Leukemia. 2003;17:2130–9. doi: 10.1038/sj.leu.2403144. [DOI] [PubMed] [Google Scholar]
- 21.Hogarth LA, Hall AG. Increased BAX expression is associated with an increased risk of relapse in childhood acute lymphocytic leukemia. Blood. 1999;93:2671–8. [PubMed] [Google Scholar]
- 22.Prokop A, Wieder T, Sturm I, Essmann F, Seeger K, Wuchter C, et al. Relapse in childhood acute lymphoblastic leukemia is associated with a decrease of the Bax/Bcl-2 ratio and loss of spontaneous caspase-3 processing in vivo. Leukemia. 2000;14:1606–13. doi: 10.1038/sj.leu.2401866. [DOI] [PubMed] [Google Scholar]
- 23.Kaparou M, Choumerianou D, Perdikogianni C, Martimianaki G, Kalmanti M, Stiakaki E, et al. Enhanced levels of the apoptotic BAX/BCL-2 ratio in children with acute lymphoblastic leukemia and high-risk features. Genet Mol Biol. 2013;36:7–11. doi: 10.1590/S1415-47572013005000003. [DOI] [PMC free article] [PubMed] [Google Scholar]


