Abstract
Hematopoietic cell transplantation (HCT) is widely performed for neoplastic and non-neoplastic diseases. HCT involves intravenous infusion of hematopoietic progenitor cells from human leukocyte antigen (HLA)-matched donor (allogeneic) or from the patient (autologous). Before HCT, the patient is prepared with high dose chemotherapy and/or radiotherapy to destroy residual malignant cells and to reduce immunologic resistance. After HCT, chemotherapy is used to prevent graft rejection and graft versus host disease (GvHD). Neurological complications are related to the type of HCT, underlying disease, toxicity of the conditioning regimens, immunosuppression caused by conditioning regimens, vascular complications generated by thrombocytopenia and/or coagulopathy, GvHD and inappropriate immune response. In this review, neurological complications are presented according to time of onset after HCT: (1) early complications (in the first month) - related to harvesting of stem cells, during conditioning (drug toxicity, posterior reversible encephalopathy syndrome), related to pancytopenia, (2) intermediate phase complications (second to sixth month) - central nervous system infections caused by prolonged neutropenia and progressive multifocal leukoencephalopathy due to JC virus, (3) late phase complications (after sixth month) - neurological complications of GvHD, second neoplasms and relapses of the original disease.
Keywords: neurological complications, hematopoietic cell transplantation, posterior reversible encephalopathy syndrome, central nervous system infections, progressive multifocal leukoencephalopathy, graft versus host disease, second neoplasm, immune reconstitution inflammatory syndrome, post-transplant acute limbic encephalitis, drug reaction with eosinophilia and systemic symptoms
Introduction
Hematopoietic cell transplantation (HCT) is an effective therapy for an expanding range of neoplastic conditions, aplastic anemia, metabolic and autoimmune diseases. The median age of patients is 10 years older than it was two decades ago and indications for transplantation may be conditions associated with significant pre-transplantation neurological deficits (Copelan, 2006; Hart and Peggs, 2007; Center for International Blood and Marrow Transplant Research, 2008; Quant and Wen, 2008; Strober et al., 2009; Passweg et al., 2014).
The long-term survival rates in children and adolescents have increased over the past 10 years and now approach 50%. Central nervous system (CNS) complications are considered an important cause of morbidity and significantly contribute to mortality after HCT (Bleggi-Torres et al., 2000; An et al., 2016; Lee et al., 2017; Maffini et al., 2017). Previous clinical studies reported neurological abnormalities in 11–59% of patients after HCT (Azik et al., 2014; Cordelli et al., 2017; Lee et al., 2017; Maffini et al., 2017). However, in autopsy studies neuropathological abnormalities were found in more than 90% of patients who died after HCT. The most common autopsy findings have been reported as subarachnoid and intraparenchymal hemorrhage with a 58.7% incidence of cerebrovascular complications, fungal infection, Wernicke encephalopathy, microglial nodular encephalopathy and toxoplasmosis (Bleggi-Torres et al., 2000). In particular, cerebrovascular bleeding, fungal infection and toxoplasmosis were found as causes of death in these studies (Bleggi-Torres et al., 2000). Mismatched related or unrelated donor allogeneic transplantation and the occurrence of graft versus host disease (GvHD) were the most significant risk factors for development of neurological complications, most probably related to pronounced immunosuppression in such patients (An et al., 2016; Lee et al., 2017; Maffini et al., 2017).
Transplantation Procedure
HCT consists of intravenous infusion of hematopoietic progenitor cells from HLA-matched donor (allogeneic) or from the patient itself (autologous) to restore the bone marrow function eradicated by high doses of chemotherapy and/or radiotherapy. The procedure generated complications related to: (1) the pre-transplantation conditioning regimen, either full intensity (myeloablative) or reduced intensity (nonmyeloblative), (2) donor type, either from a relative or unrelated human leukocyte antigen (HLA)-compatible donor (allogeneic transplantation) or from the patient prior to marrow ablation (autologous transplantation), and (3) source of stem cells (bone marrow, peripheral, or umbilical cord blood). With recognition that graft versus tumor effects are important therapeutic adjuncts, reduced intensity protocols have emerged for many hematologic disorders, and this less toxic procedure is commonly used in patients over age 50. Minimal intensity conditioning using antibody-based cell depletion is an investigational strategy currently under study for primary immunodeficiency disease (Straathof et al., 2009).
A 2012 survey collecting data from 661 centers in 48 countries reported 37,818 HCT in 33,678 patients (42% allogeneic and 58% autologous) and included 4097 pediatric patients < 18 years of age receiving HCT (71% allogeneic and 29% autologous). The proportion of allogeneic transplantation is higher in children in comparison to adults, the main indication for allogeneic transplantation in children being acute lymphoblastic leukemia (ALL) and primary immune deficiencies, while autologous transplantation has been mainly used for treating solid tumors, neuroblastoma and lymphoma (Passweg et al., 2014). In children, the vast majority of papers reported neurological complications to allogeneic HCT (Schmidt-Hieber et al., 2016).
Neurological Complications (NCs)
NCs after HCT can be classified according to: time of onset, clinical manifestations and etiology. NCs are related to the type of HCT, underlying disease, toxicity of the conditioning regimens, immunosuppression caused by conditioning regimens, vascular complications generated by thrombocytopenia and/or coagulopathy, GvHD and inappropriate immune response.
From the point of view of the onset time we can identify: (1) the early complications (the first month including HCT, conditioning transplantation procedures for HCT and the period before HCT engraftment); (2) the intermediate period complications (occurring from the second through the sixth months after transplantation); and (3) the late complications (occurring beyond six months after transplantation) (Additional Table 1 (1.3MB, tif) ). During the pre-engraftment period (< 30 days) the immune system of the patient is completely suppressed and the hematopoietic system is destroyed. Pancytopenia occurs especially associated with severe neutropenia (less than 100/mm3) causing a high risk of infections. The high dose of chemotherapy and irradiation can cause damage in mucosa, liver and other tissues. In the intermediate phase (second to sixth month) immune recovery status starts although immune depletion continues. Acute GvHD may develop, caused by alloreactive donor-derived T-cells attacking antigens in different tissues (skin, liver and intestinal tract) causing erythematous maculopapular rash, persistent anorexia, vomiting and/or diarrhea and liver dysfunction. The prophylaxis of GvHD mainly consists of post-transplant administration of immunosuppressive drugs (cyclosporine, tacrolimus, methotrexate, steroids, anti T-cell antibodies, etc.) and may impair the post-transplant immunologic reconstitution. After sixth months, immune recovery occurs progressively towards complete recovery but GvHD may develop. In chronic GvHD, patients with only liver and skin involvement have a better prognosis. Patients with extensive involvement of multiple organ systems may experience prolonged cellular and humoral immunosuppression associated with the immunosuppressive regimens necessary to control GvHD and the prognosis may be poor. Graft failure or relapse of the underlying disease may occur during this phase (Schmidt-Hieber et al., 2016).
Neurological complications of hematopoietic cell transplantation.
Early neurologic complications (in the first month)
Complications related to harvesting of stem cells
Prior to cell harvesting, during administration of hematopoietic growth factors, patients with preexisting autoimmune diseases such as multiple sclerosis may experience exacerbations of their underlying disease (Openshaw et al., 2000; Burt et al., 2001). In children as well as in adults, the use of cytotoxic chemotherapy (cyclophosphamide) in addition to growth factors in the cell mobilization regimen reduces this risk of disease relapse although can be associated with increased risk of infection (Burt et al., 2001; Uy et al., 2015).
During the harvesting of stem cells, complications are very rare, such as an accidental entry into the subarachnoid space leading to a syndrome of intracranial hypotension.
Complications during conditioning
Although allogeneic hematopoietic cell transplants have been generally thought to have higher risk of adverse effects, a large retrospective study of 425 adult patients demonstrated a similar incidence of neurologic problems in autologous and allogeneic HCT recipients (Rosenfeld and Pruitt, 2006).
On the contrary, in children allogeneic HCT was associated more frequently than autologous HCT with neurologic complications and increased morbidity and mortality due to the immunosuppression toxicity used to prevent GvHD and mismatched transplantation, primary disease being acute myeloid leukemia (AML), older age and the presence of grade II or higher GvHD (Maher et al., 2017).
Most complications during conditioning have been attributed to high-dose chemotherapy, prophylactic drugs against GvHD, total body irradiation and antibiotics used for prophylaxis or treatment of infections. Drug-to-drug interactions and the combination of different neurotoxicities of those drugs that are administered simultaneously play a pivotal role in the appearance of neurologic complications.
The most common of the NCs produced by the preparative regimens are: seizures (busulfan - the estimated incidence of seizures is 10% and with use of antiepileptic drug prophylaxis only 1.3%, cytarabine, melphalan, ifosfamide), severe encephalopathy and posterior reversible encephalopathy syndrome (PRES) (carboplatin, carmustine, cyclophosphamide, etoposide, fludarabine, ifosfamide, melphalan), peripheral neuropathy (carboplatin, etoposide) and ototoxicity (carboplatin), pancerebellar syndrome (cytarabine), lymphocytic meningitis (cytarabine, thiotepa), progressive multifocal leukoencephalopathy (PML) (monoclonal antibodies such as alemtuzumab or rituximab, fludarabine), necrotizing microangiopathy, additive toxicity with cranial irradiation, transient stroke-like episodes with diffusion weighted imaging-magnetic resonance imaging (DWI-MRI) abnormalities, transverse myelitis (methotrexate), Listeria myelitis, cytomegalovirus (CMV) encephalitis, immune reconstitution inflammatory syndrome (IRIS), Guillain-Barré syndrome (alemtuzumab) (Pruitt et al., 2013).
Antibiotics used in infection prophylaxis or treatment of infectious complications may also produce NCs: seizures (acyclovir, cefepime, imipenem), parkinsonism and confusion (amphotericin B), encephalopathy (acyclovir, cefepime), myoclonus (cefepime), ischemic optic neuropathy, PRES, serotonin syndrome, neuropathy (linezolid, posaconazole enhances vincristine neuropathy), reversible cerebellar disease, sensorimotor peripheral neuropathy, optic neuropathy and autonomic dysfunction (metronidazole) (Hobson-Webb et al., 2006; Patel et al., 2008), visual hallucinations (voriconazole). There are several reports on neurotoxic effects of carbapenemics particularly imipenem/cilastatin (Norrby, 2000). Quinolone neurotoxicity may manifest with seizures, encephalopathy, myoclonus and toxic psychosis (Isaacson et al., 1993).
Chemotherapy used for post-transplant GvHD prophylaxis have been reported to produce the following NCs: tremor (cyclosporine), PRES (cyclosporine, tacrolimus, sirolimus), progressive multifocal leukoencephalopathy (PML) (mycophenolate), chronic inflammatory demyelinating polyneuropathy (CIDP) (cyclosporine, tacrolimus), mutism, pseudotumor cerebri (cyclosporine), brachial plexopathy, optic neuropathy, hearing loss (tacrolimus), seizures (cyclosporine, tacrolimus) (Kang et al., 2015). Seizures and encephalopathy are most common immediately after the actual hematopoietic cell infusion; seizures are typically generalized and do not recur, even without antiepileptic drugs. This is probably related to toxicity from dimethyl sulfoxide used as a cryopreservative and may be associated with diffuse white matter changes on magnetic resonance imaging (MRI) that resolve over days to weeks (Higman et al., 2000; Bauwens et al., 2005). Many patients experience delirium without focal neurologic deficits within 30 days of HCT (Fann et al., 2002; Fann et al., 2007). Risk factors for development of delirium include elevated pre-transplant levels of alkaline phosphatase and blood urea nitrogen, as well as post-transplant use of opiates (Fann et al., 2011).
Among the most important and reversible conditions that may occur is PRES (Figure 1). Also known as reversible posterior leukoencephalopathy syndrome, PRES is most common during this early post-transplant period but risk continues throughout the patient's course. Calcineurin inhibitors such as cyclosporine and tacrolimus are the major offenders, but PRES has been described with sirolimus and everolimus as well as with dexamethasone (Nguyen et al., 2009; Shkalim-Zemer et al., 2017; Zama et al., 2018). PRES is manifested by: acute mental status changes, cortical blindness, hypertension and seizures and MRI may show petechial, lobar or subarachnoid hemorrhage. The syndrome results from dysregulation of cerebral vasculature, leading to vasogenic edema preferentially but not exclusively, affecting the parieto-occipital regions. Brainstem, basal ganglia, and spinal cord involvement all have been reported as well as status epilepticus (McKinney et al., 2007; Briganti et al., 2009; Tambasco et al., 2016). Two theories have been formulated to explain the development of cerebral vasogenic edema: the first theory incriminates hypertension suggesting that rapidly increasing blood pressure produce cerebral hyperperfusion and damage of the capillary bed with leakage of the fluid in the interstitium, and the second theory point to the endothelial cell activation with subsequent cerebral vasoconstriction and hypoperfusion (Cordelli et al., 2017). The development of PRES with the use of one immunosuppressive drug does not preclude safe use of another related agent. A clinical dilemma is related to the appropriate choice and duration of antiepileptic drug therapy. The use of nonenzyme-inducing antiepileptic therapy has been proposed during and for one month following the acute episode of PRES if accompanied by clinical seizures.
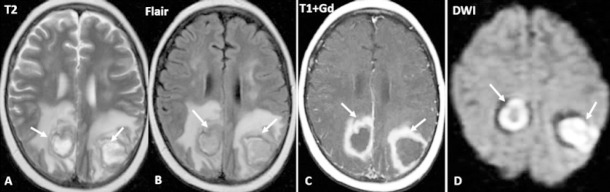
Figure 1.
Brain MRI of a 32-year-old female with Hodgkin lymphoma treated with allogeneic hematopoietic cell transplantation on March 23, 2018.
Posterior reversible encephalopathy syndrome (PRES) typical magnetic resonance imaging (MRI) aspect (black arrows)- symmetrical areas of T2 and Flair hyperintensity in both parieto-occipital lobes. Multiple supratentorial hemosiderotic spots (white arrow).
Complications of pancytopenia
Thrombocytopenia and coagulation disturbances generate vascular complications. Subdural hematoma is the most common vascular complication, but intraparenchymal hemorrhage (IPH), subarachnoid hemorrhage, cerebral venous thrombosis and infarction may occur (Figure 2). In adults, autopsy series suggest an incidence of subdural hematoma up to 11.9% (Mohrmann et al., 1990) while retrospective clinical reviews have documented this complication in 2% to 5.7% of patients (Najima et al., 2009; Zhang et al., 2016).
Figure 2.
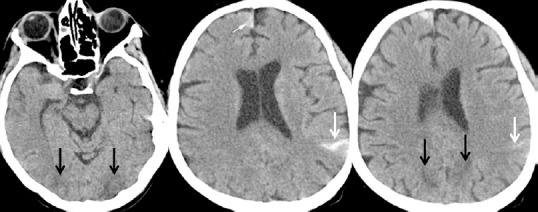
Brain CT-scan of a 32-year-old female with Hodgkin lymphoma treated with allogeneic hematopoietic cell transplantation on March 23, 2018.
Parieto-occipital bilateral hypodensities (black arrows). Small hemorrhagic left parietal and right frontal SAH accumulations (white arrow).
A retrospective review of 622 allogeneic HCT recipients (Najima et al., 2009), over a period of 20 years identified a total of 21 cases (3.4%) including 15 cases of intraparenchymal hemorrhage (IPH), two cases of subarachnoid hemorrhage (SAH), and four cases of subdural hematoma (SDH). The median time from transplantation to the onset of ICH was 63 days (range 6-3488 days). The clinical features of post-transplant intracerebral hemorrhage patients were similar and included hypertension, diabetes mellitus, chronic GvHD, systemic infection, and veno-occlusive disease (VOD), recently referred to as sinusoidal obstruction syndrome, in addition to severe thrombocytopenia. Mortality rate was especially high (89%) after IPH with a median survival of 2 days (range 0–148 days). In contrast, all patients with SAH or SDH following HCT survived. The cause of post-transplant ICH appears to be multifactorial, including thrombocytopenia, hypertension, acute GvHD, VOD, and radiation therapy. In children, intracranial hemorrhage has been reported as life-threatening complication after HCT in a small number of patients (Uckan et al., 2005) and thrombotic disease is reported in association with endocarditis, indwelling catheter and characteristically with treatment with L-asparaginase in acute lymphoblastic leukemia (ALL) (Caruso et al., 2006).
Coagulation deficits generates hepatic veno-occlusive disease also called sinusoidal obstruction syndrome, that may present with encephalopathy, fluid retention and rising bilirubin (Caruso et al., 2006).
Neutropenia may cause infections due to viruses, bacteria, fungi and parasites. Clinical infectious manifestations may be absent due to host's immunosuppression, but CNS infection should be suspected upon occurrence of new neurological signs, fever or systemic, particularly pulmonary, infection (Figures 3–5). Nosocomial infections may be caused by methicillin-resistant Staphylococcus aureus, multiresistant enterococci, and gram-negative organisms, while intravenous lines may be a potential source of infection with Candida (Cesaro et al., 2018). CNS candidiasis involves vasculitis, hemorrhage and, less commonly, thrombotic infarction and it comprises multiple ring-enhancing micro-abscesses at the cortico/medullary junction, cerebellum, and basal ganglia (Lai et al., 1997).
Figure 3.
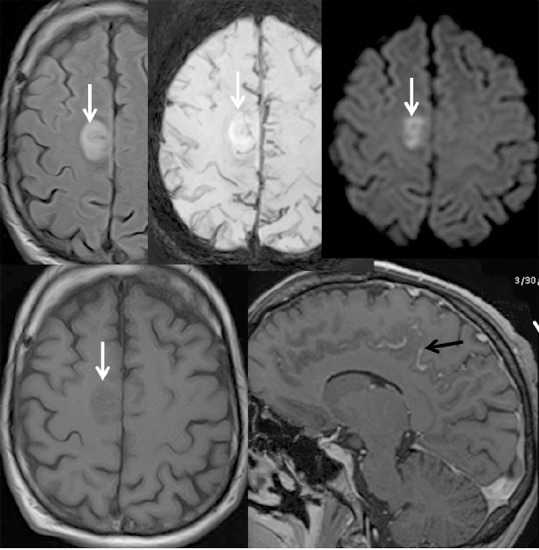
Brain MRI of a 30-year-old male with acute myeloid leukemia who received allogeneic hematopoietic cell transplantation on December 19, 2017.
Right frontal cortical parasagital lesion with “acute” pattern (restriction of water on diffusion weighted imaging (DWI))- (white arrow) and leptomeningeal enhancement (black arrows).
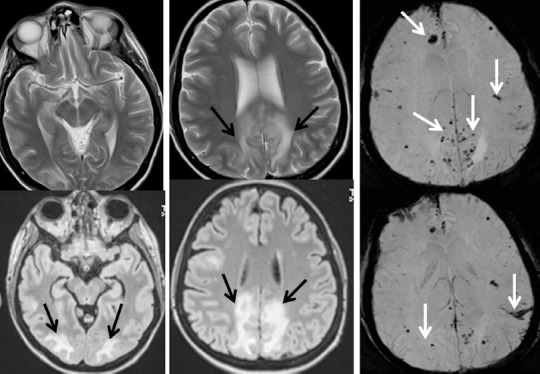
Figure 5.
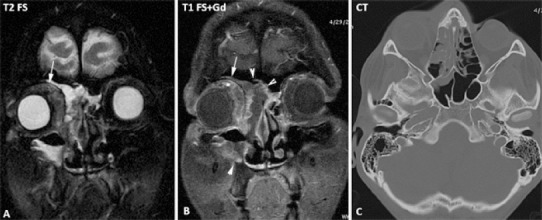
Brain MRI of a male treated with allogeneic hematopoietic cell transplantation in 2010.
Right orbital cellulitis: magnetic resonance imaging (MRI) and CT aspects: oedema and inflammation of the periorbital and orbital contents (white arrow); right invasive maxillo-ethmoido-frontal sinusitis (head arrows)
Figure 4.
Brain MRI of a 12-year-old male with acute lymphoblastic leukemia treated with allogeneic hematopoietic cell transplantation.
Cerebral abscesses located into parieto-occipital regions: typical magnetic resonance imaging (MRI) aspects-ring-enhancing lesions, with high signal intensity on T2-weighted images and low signal intensity on T1-weighted images.
In children, the predominant fungal pathogen is Aspergillus spp., with Aspergilus fumigatus prevailing over other species with an annual incidence of 0.4% of hospitalized immunocompromised children (Zaoutis et al., 2006; McDonald, 2010; Groll et al., 2014; Cordelli et al., 2017). Although culture from a sterile site remain the gold standard for diagnosis and provide antifungal susceptibilities information, invasive procedures are needed, and the diagnostic yield is low. Since Aspergillus is rarely recovered from blood cultures (Qualter et al., 2014; Cordelli et al., 2017), in many patients the diagnosis relies on indirect findings including fungal markers (galactomannan antigen and 1,3-β-d-glucan) and neuroimaging findings. Typical neuroimaging features include ring-enhancing lesions, with a central T2 hypointensity area, associated with hemorrhagic foci and peculiar intracavitary projection at the diffusion study, representing fungal hypae (Luthra et al., 2007; Cordelli et al., 2017) mainly located to thalami, basal ganglia, and corpus callosum and subcortical regions, as Aspergillus has a peculiar “philia” for perforating arteries. Due to its angioinvasive nature, arterial infarction (lacunar and territorial), vasculitis, and mycotic aneurysm can be encountered (Yamada et al., 2002; Cordelli et al., 2017). Treatment includes amphotericin B lipid formulations and azoles (Kleinschmidt-DeMasters, 2002; Schwartz et al., 2005; Dotis et al., 2007; Cordelli et al., 2017). Prognosis correlates primarily with immune status, with allo-HCT patients having sixfold greater odds of death (Burgos et al., 2008; Cordelli et al., 2017).
Disseminated Rhiszopus microspores infection, Rhizomucor pussilus infection zycomycosis, mucormycosis and Lichteimia ramose infection have been also reported in children after HCT particularly for aplastic anemia (Rawlinson et al., 2011; Ye et al., 2013; Pana et al., 2016).
In adult recipients of HCT, another cause of CNS infection is Listeria monocytogenes that produces brain abscess, with a decreasing trend because of the use of trimethoprim–sulfamethoxazole and Nocardia asteroids (Safdar et al., 2002). In children, Listeria monocytogenes brain abcesses are rarely reported (Viscoli et al., 1991).
Cryptococal meningitis is typical for HIV-infected patients and it is very rarely reported in HCT recipients. Toxoplasma gondii allograft transmission is most strongly associated with cardiac transplantation but among HCT recipients the rates vary from 0.3% to 13% and are higher in countries where toxoplasmosis is more prevalent (Martino et al., 2000; Fricker-Hidalgo et al., 2009). Neurotoxoplasmosis presents with multiple abscesses in the white and gray matter of cerebral hemispheres associated with ring enhancement on CT and MRI. Detection of toxoplasma-DNA by polymerase chain reaction (PCR) in cerebro-spinal fluid (CSF) and stereotactic biopsy with histologic confirmation establish the diagnosis. Trimetroprim-sulphametoxazole (TMP-SFX) associated with clyndamicin or pyrimethamine is the current therapy of choice and prevention therapy with antiprotozoal molecules have been recommended.
Frequent viral infections are caused by herpesviridae (herpes simplex, Epstein-Barr virus, varicella zoster virus, cytomegalovirus (CMV) and HHV-6) and polyomaviridae (JC polyomavirus – JCV). Extensive chronic GvHD is reported to be associated with an increased rate of viral infections (Verdeguer et al., 2010). Appropriate prophylaxis has delayed the onset of such infections but reactivation of CMV has been associated with an increased risk of Listeria meningitis that might present as diffuse cerebritis or rhombencephalitis (Safdar et al., 2002).
CMV affects both peripheral and central nervous system, chorioretinitis and myelitis-radiculitis followed by encephalitis and ventriculitis being the most common forms of neurologic involvement. Ganciclovir and foscarnet are the most effective first line agents for CMV infection after HCT. HHV-6 primary infection may be asymptomatic or may cause a febrile exanthema (roseola infantum). The virus is latent in the salivary gland, white blood cells and the brain and may express as encephalitis characterized by temporal lobe seizures, fever and mental status changes including insomnia. Diagnosis is made by PCR analysis for HHV-6 in CSF and confirmation of HHV-6B protein by immunohistochemistry. Brain MRI usually shows confluent hyperintense lesion on T2 sequences and no enhancement on T1 sequences involving the mesial temporal lobes and limbic system. However acute necrotizing encephalopathy with symmetric involvement of the basal ganglia, thalamus and brainstem may occur (Ohsaka et al., 2006). Also, reactivation of preexisting neurocysticercosis has been described (Teive et al., 2008). Donor derived infections like hepatitis B, C, CMV to neurologically specific viral contaminations such as adenovirus or Coxsackie virus B4 that produce encephalitis may be fatal (Cree et al., 2003; Gigliotti et al., 2003; Zekri et al., 2004). At 2 to 4 weeks after engraftment may appear fever, rash and headache, as the absolute neutrophil count exceeds 500.
Another neurologic complication is post-transplant acute limbic encephalitis (PALE) characterized by anterograde amnesia, the syndrome of inappropriate antidiuretic hormone secretion, mild CSF pleocytosis and EEG abnormalities with or without clinical seizures (Seeley et al., 2007). The most common cause of this syndrome is HHV-6, usually due to a reactivation of host infection. The disease is frequently associated to severe GvHD due to unrelated donors or conditioning regimens containing antithymocyte globulin, sirolimus, interleukin 12 antibodies and especially in patients receiving alemtuzumab. PALE treatment is represented by antiepileptic therapy, ganciclovir and foscarnet.
A syndrome that resembles PALE is drug reaction (or rash) with eosinophilia and systemic symptoms (DRESS). DRESS is characterized by skin eruption, fever, lymphadenopathy, syndrome of inappropriate antidiuretic hormone secretion with hyponatremia and multiorgan involvement and can be triggered by sulfonamides and antiepileptic drugs. DRESS is associated with reactivation of CMV, EBV and HHV-1, 6, 7 and may resemble GvHD. Skin biopsy helps to differentiate the two conditions (Seishima et al., 2006).
Immune reconstitution inflammatory syndrome (IRIS) may occur during engraftment, but has also been reported later after HCT and even several months after discontinuation of immunosuppression. IRIS is caused by an immune rebound leading to Th1 immune response against infectious antigens and in some cases unknown antigens. Alemtuzumab, an anti-CD52 monoclonal antibody, has been reported to increase the risk of IRIS with both systemic (Graves disease) and neurological involvement after initial cryptococcal meningitis treatment.
IRIS should be considered when a patient has had an infection and there are (1) new or worsening clinical or radiologic manifestations consistent with an inflammatory process; (2) symptoms during receipt of appropriate antibiotic therapy that cannot be explained by newly acquired infection; and (3) negative results of cultures or stable or reduced biomarkers for the initial pathogen during the diagnostic workup for the inflammatory process (Ingram et al., 2007; Airas et al., 2010).
Intermediate phase neurologic complications (second to sixth month)
The most common complications of the intermediate phase are: central nervous system infections caused by prolonged neutropenia and progressive multifocal leukoencephalopathy (PML) due to JC virus. Rare cases were reported with neurologic complications such as: acute and chronic demyelinating polyneuropathy and immune mediated demyelinating disease.
Invasive opportunistic fungal diseases are important causes of morbidity and mortality in pediatric patients who have had an allogeneic HCT (Groll et al., 2014). Aspergillosis is one of the most common infections in allogeneic HCT group (Ingram et al., 2007; Airas et al., 2010), the risk reaching 10%, especially for patients receiving conditioning regimens with fludarabine and alemtuzumab. The usual route of infection is respiratory with pulmonary and paranasal sinus disease. Hematogenous spread leads to mycotic aneurysm, vasculitis and subarachnoid hemorrhage. Multiple septic infarcts may occur, involving the lenticulostriate and thalamoperforating arteries associated with hemorrhage and abscess formation. The areas commonly involved are basal ganglia, thalamus, corpus callosum and brainstem. There may be little or no pleocytosis in the CSF and daily serum galactomannan testing has been used to establish when to use preemptive therapy (Maertens et al., 2005). CNS aspergillosis may be treated with amphotericin B, voriconazole or caspofungin but mortality rates remain high.
Toxoplasma gondii allograft transmission may be present in HCT patients, with a rate of 2.94%, especially in countries where toxoplasmosis is more prevalent. Mortality rate is very high in cerebral toxoplasmosis and the occurrence is reduced by use of trimethoprim-sulfamethoxazole prophylaxis (Hakko et al., 2013).
Viral infections are other important causes of mortality and morbidity in children receiving HCT. Activation of latent herpes virus type 6 and 7 (HHV-6 and HHV-7) were reported to be common in pediatric HCT. Most infections were self-limited and were associated with adenovirus infection and severe GvHD (Fule Robles et al., 2014). Encephalitis due to primary HHV-6B infection in young children is commonly reported in Japan, but very rarely elsewhere, suggesting a genetic predisposition. Limbic encephalitis due to reactivation of HHV-6B was reported in HCT pediatric patients, particularly after receipt of cord blood. It has a poor outcome, preventive strategies being ineffective. In children receiving HCT, cytomegalovirus reactivation was associated with increased relapse rate; accelerated overall immune recovery rather than CMV-driven immunity had a favorable outcome impact on relapse rate (Jeljeli et al., 2014). In a study which included 2628 patients after allogeneic HCT, viral encephalitis was reported as being caused by HHV-6 (28%), Epstein-Barr virus (19%), herpes simplex virus (13%), JC virus (9%), varicella zoster virus (6%) and adenovirus (3%). In 16% of patients more than one virus was identified. The use of OKT-3 and alemtuzumab was associated with increased risk of viral encephalitis after allogeneic HCT (Schmidt-Hieber et al., 2011; Yuan et al., 2018). Progressive multifocal leukoencephalopathy, a subacute demyelinating disorder caused by JC virus has been associated with fludarabine and rituximab therapy in transplanted population. Symptoms typically develop more than one month after HCT and have been reported as late as several years post-transplantation at a time of apparent minimal immunosuppression (Gheuens et al., 2010). Long-term immunosuppression in patients who underwent transplantation from alternative donors, such as mismatched unrelated donors or umbilical cord blood, represents a cause of very high risk of infection (Servais et al., 2014).
Peripheral nervous system involvement has been reported in the intermediate phase after HCT. Guillain-Barre syndrome (acute polyradiculoneuritis) was reported in three children who received allogeneic HCT and conditioning regimen containing high dose cytosine arabinoside therapy (Rodriguez et al., 2002). In contrast with the report of human CMV encephalitis in adult, in children it was reported a CMV radiculopathy with good outcome (Colombo et al., 2012).
Immune mediated demyelinating disease (IMDD) have been reported mostly in adults after allogeneic HCT. In a study including 1484 transplanted patients 7 patients (0.5%) suffered from IMDD, the median time from transplant to neurologic symptoms being 120 days (range 60–390 days). Three patients had acute demyelinating encephalomyelitis (ADEM), three acute inflammatory demyelinating polyradiculopathy (AIDP) and one autonomic neuropathy. Patients were treated with IV immunoglobulin, high-dose steroids and/or rituximab and five patients had a significant recovery (Delios et al., 2012).
Late phase neurologic complications (after sixth months)
In the late phase after HCT the possible neurologic complications are: CNS relapses of the original disease, neurologic complications of GvHD and second neoplasms.
GvHD occurs after allogeneic HCT in two major forms: acute (< 100 days post-transplant) and chronic (> 100 days). It most frequently involves skin, liver, gastrointestinal tract, eyes and peripheral nerves (Jagasia et al., 2015).
Neurological manifestation of chronic GvHD are rare and can affect both peripheral and central nervous system. They usually occur several months to years after HCT after administration of multiple potentially neurotoxic drugs, when often infectious and metabolic complications have occurred.
Manifestations of peripheral nervous system, neuromuscular junction and muscle involvement in GvHD include polymyositis, myasthenia gravis (MG) and chronic inflammatory demyelinating polyneuropathy (CIDP) often developing late after HCT at a time of reduction in immunosuppressive therapy (Kamble et al., 2007; Grauer et al., 2010).
Chronic GvHD-related myositis appears to be similar to idiopathic polymyositis in its clinicopathological presentation (Sharaf and Prayson, 2014), especially in juvenile polymyositis where maternal microchimerism has been demonstrated (Tse et al., 1999).
Myasthenia gravis is rarely reported post-HCT in children and have a severe clinical presentation (Tse et al., 1999). There is one report of myasthenia gravis in a 2 years and 10 months old female with Griscelli syndrome who developed severe MG at 22 months post-HCT. She was unresponsive to cyclosporine A, methylprednisolone, intravenous immunoglobulin, and mycophenolate mofetil and the symptoms could only be controlled after plasma exchange and subsequent use of rituximab, in addition to cyclosporine A and mycophenolate mofetil maintenance (Unal et al., 2014).
CNS involvement in GvHD is quite rare and occurs almost exclusively in adults during chronic GvHD, requiring differentiation from B-cell lymphoproliferative processes. Neurologic presentation is highly variable and may include: vasculitis with angiographic manifestations of dilated and narrowed arteries and aneurysms leading to parenchymal hemorrhage (Ma et al., 2002), focal lymphocytic encephalitis sometimes with aspect of mass lesion (inflammatory pseudotumor) (Tsutsumi et al., 2005), acute demyelinating encephalomyelitis (Tomonari et al., 2003) or relapsing-remitting multiple sclerosis (Armstrong et al., 2010) and T-cell meningitis with high protein in CSF and non-specific abnormalities on MRI (Kyllerman et al., 2008).
Recent studies analyzing cohorts with a high number of pediatric recipients of HCT showed increased risk of second neoplasms (SNs) among all primary childhood cancer cases (Meadows et al., 2009; Danner-Koptik et al., 2012). When compared to the general population, the overall standardized incidence ratio of developing SNs was 6.4 with an estimated 30-year cumulative incidence of 9.3%. The use of high dose chemotherapy to eradicate disease in these aggressive pediatric malignancies, specifically alkylating agents, anthracyclines, and epipodophyllotoxins increased the risk of SNs. Radiation has also been shown to increase the risk of SNs. It has been shown that there is a close relationship between the risk of SNs and radiation dose in childhood cancer survivors (Hijiya et al., 2007). Central nervous system (CNS) SNs, specifically subsequent gliomas and meningiomas, have been associated with prior radiation therapy (Walter et al., 1998; Majhail et al., 2011). Allogeneic hematopoietic stem cell transplantation also increases the risk for SNs in children (Wong et al., 2009). Even for children who received autologous HCT, the risk of developing SNs is 24 times higher than in the general population and the types of tumors reported were: neuroblastoma (39%), lymphoma (26%), sarcoma (18%), CNS tumors (14%) and Wilms tumor (2%) after a median follow-up of 8 years.
There is also an important concern about cognitive impairment due to total body irradiation,
GvHD treatments, and cytotoxic agents. A recent prospective study examined cognitive changes in 284 adult patients 2 years after HCT. Allogeneic HCT recipients demonstrated worse scores in processing speed, executive function and verbal fluency compared with autologous recipients. Only two-thirds of all patients were working part time or full time one year after diagnosis, although employment status clearly depended on the underlying disease. However, the issue of cognitive impairment after HCT was not studied in pediatric patients.
Conclusion
Neurological complications are an important cause of morbidity and mortality in children and adults who underwent allogeneic as well as autologous HCT. They are mainly related to CNS toxicity of immunosuppressive drugs used for preconditioning regimens, to pancytopenia following chemotherapy as well as CNS infections that represent a major challenge being associated to high mortality. The peripheral nervous system involvement, manifested as acute polyradiculoneuritis, radiculopathies and polyneuropathies, was also reported. Neuromuscular junction and muscle pathology were present in some cases of GVHD. Several studies analyzing cohorts showed that the incidence of developing secondary neoplasms is increased in pediatric patients who received HCT when compared with general population. Although domain-specific neurocognitive deficits were reported in adult patients receiving HCT, cognitive impairment was not studied in pediatric patients. All these complications preferentially occur at specific intervals after HCT and neurologist must recognize them in order to promptly diagnose and apply appropriate treatment.
Additional file:
Additional Table 1 (1.3MB, tif) : Neurological complications of hematopoietic cell transplantation.
Acknowledgments
Author thanks Dr. Ioan Cristian Lupescu (Neurology Department, Fundeni Clinical Institute, Romania) for rearranging the references and Mrs. Monica Marinescu (Freelancer, collaborator of Neurology Department Fundeni Clinical Institute, Romania) for the proofreading of the English version of the manuscript.
Footnotes
Conflicts of interest: None declared.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- Airas L, Päivärinta M, Röyttä M, Karhu J, Kauppila M, Itälä-Remes M, Remes K. Central nervous system immune reconstitution inflammatory syndrome (IRIS) after hematopoietic SCT. Bone Marrow Transplant. 2010;45:593–596. doi: 10.1038/bmt.2009.186. [DOI] [PubMed] [Google Scholar]
- An K, Wang Y, Li B, Luo C, Wang J, Luo C, Chen J. Prognostic factors and outcome of patients undergoing hematopoietic stem cell transplantation who are admitted to pediatric intensive care unit. BMC Pediatr. 2016;16:138. doi: 10.1186/s12887-016-0669-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RJ, Elston JS, Hatton CS, Ebers GC. De novo relapsing-remitting multiple sclerosis following autologous stem cell transplantation. Neurology. 2010;75:89–91. doi: 10.1212/WNL.0b013e3181e6215d. [DOI] [PubMed] [Google Scholar]
- Azik F, Yazal Erdem A, Tavil B, Bayram C, Tunc B, Uckan D. Neurological complications after allogeneic hematopoietic stem cell transplantation in children, a single center experience. Pediatr Transplant. 2014;18:405–411. doi: 10.1111/petr.12265. [DOI] [PubMed] [Google Scholar]
- Bauwens D, Hantson P, Laterre PF, Michaux L, Latinne D, De Tourtchaninoff M, Cosnard G, Hernalsteen D. Recurrent seizure and sustained encephalopathy associated with dimethylsulfoxide-preserved stem cell infusion. Leuk Lymphoma. 2005;46:1671–1674. doi: 10.1080/10428190500235611. [DOI] [PubMed] [Google Scholar]
- Bleggi-Torres LF, de Medeiros BC, Werner B, Neto JZ, Loddo G, Pasquini R, de Medeiros CR. Neuropathological findings after bone marrow transplantation: an autopsy study of 180 cases. Bone Marrow Transplant. 2000;25:301–307. doi: 10.1038/sj.bmt.1702140. [DOI] [PubMed] [Google Scholar]
- Briganti C, Caulo M, Notturno F, Tartaro A, Uncini A. Asymptomatic spinal cord involvement in posterior reversible encephalopathy syndrome. Neurology. 2009;73:1507–1508. doi: 10.1212/WNL.0b013e3181bf98c9. [DOI] [PubMed] [Google Scholar]
- Burgos A, Zaoutis TE, Dvorak CC, Hoffman JA, Knapp KM, Nania JJ, Prasad P, Steinbach WJ. Pediatric invasive aspergillosis: a multicenter retrospective analysis of 139 contemporary cases. Pediatrics. 2008;121:e1286–1294. doi: 10.1542/peds.2007-2117. [DOI] [PubMed] [Google Scholar]
- Burt RK, Fassas A, Snowden J, van Laar JM, Kozak T, Wulffraat NM, Nash RA, Dunbar CE, Arnold R, Prentice G, Bingham S, Marmont AM, McSweeney PA. Collection of hematopoietic stem cells from patients with autoimmune diseases. Bone Marrow Transplant. 2001;28:1–12. doi: 10.1038/sj.bmt.1703081. [DOI] [PubMed] [Google Scholar]
- Caruso V, Iacoviello L, Di Castelnuovo A, Storti S, Mariani G, de Gaetano G, Donati MB. Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients. Blood. 2006;108:2216–2222. doi: 10.1182/blood-2006-04-015511. [DOI] [PubMed] [Google Scholar]
- Center for International Blood and Marrow Transplant Research (2008) Progress Report: January-December. Milwaukee, WI: CIBMTR; 2007. [Google Scholar]
- Cesaro S, Tridello G, Blijlevens N, Ljungman P, Craddock C, Michallet M, Martin A, Snowden JA, Mohty M, Maertens J, Passweg J, Petersen E, Nihtinen A, Isaksson C, Milpied N, Rohlich PS, Deconinck E, Crawley C, Ledoux MP, Hoek J, et al. Incidence, risk factors and long-term outcome of acute leukemia patients with early candidemia after allogeneic stem cell transplantation. a study by the acute leukemia and infectious diseases working parties of EBMT. Clin Infect Dis. 2018 doi: 10.1093/cid/ciy150. doi: 10.1093/cid/ciy150. [DOI] [PubMed] [Google Scholar]
- Colombo AA, Giorgiani G, Rognoni V, Villani P, Furione M, Bonora MR, Alessandrino EP, Zecca M, Baldanti F. Differential outcome of neurological HCMV infection in two hematopoietic stem cell transplant recipients. BMC Infect Dis. 2012;12:238. doi: 10.1186/1471-2334-12-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- Cordelli DM, Masetti R, Zama D, Toni F, Castelli I, Ricci E, Franzoni E, Pession A. Central nervous system complications in children receiving chemotherapy or hematopoietic stem cell transplantation. Frontiers in pediatrics. 2017;5:105. doi: 10.3389/fped.2017.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree BC, Bernardini GL, Hays AP, Lowe G. A fatal case of coxsackievirus B4 meningoencephalitis. Arch Neurol. 2003;60:107–112. doi: 10.1001/archneur.60.1.107. [DOI] [PubMed] [Google Scholar]
- Danner-Koptik KE, Majhail NS, Brazauskas R, Wang Z, Buchbinder D, Cahn JY, Dilley KJ, Frangoul HA, Gross TG, Hale GA, Hayashi RJ, Hijiya N, Kamble RT, Lazarus HM, Marks DI, Reddy V, Savani BN, Warwick AB, Wingard JR, Wood WA, et al. Second malignancies after autologous hematopoietic cell transplantation in children. Bone Marrow Transplant. 2012;48:363. doi: 10.1038/bmt.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delios AM, Rosenblum M, Jakubowski AA, DeAngelis LM. Central and peripheral nervous system immune mediated demyelinating disease after allogeneic hemopoietic stem cell transplantation for hematologic disease. J Neurooncol. 2012;110:251–256. doi: 10.1007/s11060-012-0962-9. [DOI] [PubMed] [Google Scholar]
- Dotis J, Iosifidis E, Roilides E. Central nervous system aspergillosis in children: a systematic review of reported cases. Int J Infect Dis. 2007;11:381–393. doi: 10.1016/j.ijid.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Fann JR, Roth-Roemer S, Burington BE, Katon WJ, Syrjala KL. Delirium in patients undergoing hematopoietic stem cell transplantation. Cancer. 2002;95:1971–1981. doi: 10.1002/cncr.10889. [DOI] [PubMed] [Google Scholar]
- Fann JR, Alfano CM, Roth-Roemer S, Katon WJ, Syrjala KL. Impact of delirium on cognition, distress, and health-related quality of life after hematopoietic stem-cell transplantation. J Clin Oncol. 2007;25:1223–1231. doi: 10.1200/JCO.2006.07.9079. [DOI] [PubMed] [Google Scholar]
- Fann JR, Hubbard RA, Alfano CM, Roth-Roemer S, Katon WJ, Syrjala KL. Pre- and post-transplantation risk factors for delirium onset and severity in patients undergoing hematopoietic stem-cell transplantation. J Clin Oncol. 2011;29:895–901. doi: 10.1200/JCO.2010.28.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker-Hidalgo H, Bulabois CE, Brenier-Pinchart MP, Hamidfar R, Garban F, Brion JP, Timsit JF, Cahn JY, Pelloux H. Diagnosis of toxoplasmosis after allogeneic stem cell transplantation: results of DNA detection and serological techniques. Clin Infect Dis. 2009;48:e9–e15. doi: 10.1086/595709. [DOI] [PubMed] [Google Scholar]
- Fule Robles JD, Cheuk DK, Ha SY, Chiang AK, Chan GC. Human herpesvirus types 6 and 7 infection in pediatric hematopoietic stem cell transplant recipients. Ann Transplant. 2014;19:269–276. doi: 10.12659/AOT.889995. [DOI] [PubMed] [Google Scholar]
- Gheuens S, Pierone G, Peeters P, Koralnik IJ. Progressive multifocal leukoencephalopathy in individuals with minimal or occult immunosuppression. J Neurol Neurosurg Psychiatry. 2010;81:247–254. doi: 10.1136/jnnp.2009.187666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliotti AR, Fioredda F, Giacchino R. Hepatitis B and C infection in children undergoing chemotherapy or bone marrow transplantation. J Pediatr Hematol Oncol. 2003;25:184–192. doi: 10.1097/00043426-200303000-00002. [DOI] [PubMed] [Google Scholar]
- Grauer O, Wolff D, Bertz H, Greinix H, Kuhl JS, Lawitschka A, Lee SJ, Pavletic SZ, Holler E, Kleiter I. Neurological manifestations of chronic graft-versus-host disease after allogeneic haematopoietic stem cell transplantation: report from the Consensus Conference on Clinical Practice in chronic graft-versus-host disease. Brain. 2010;133:2852–2865. doi: 10.1093/brain/awq245. [DOI] [PubMed] [Google Scholar]
- Groll AH, Castagnola E, Cesaro S, Dalle JH, Engelhard D, Hope W, Roilides E, Styczynski J, Warris A, Lehrnbecher T. Fourth European Conference on Infections in Leukaemia; Infectious Diseases Working Party of the European Group for Blood Marrow Transplantation (EBMT-IDWP); Infectious Diseases Group of the European Organisation for Research and Treatment of Cancer (EORTC-IDG); International Immunocompromised Host Society (ICHS); European Leukaemia Net (ELN) (2014) Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or allogeneic haemopoietic stem-cell transplantation. Lancet Oncol. 15:e327–340. doi: 10.1016/S1470-2045(14)70017-8. [DOI] [PubMed] [Google Scholar]
- Hakko E, Ozkan HA, Karaman K, Gulbas Z. Analysis of cerebral toxoplasmosis in a series of 170 allogeneic hematopoietic stem cell transplant patients. Transpl Infect Dis. 2013;15:575–580. doi: 10.1111/tid.12138. [DOI] [PubMed] [Google Scholar]
- Hart DP, Peggs KS. Current status of allogeneic stem cell transplantation for treatment of hematologic malignancies. Clin Pharmacol Ther. 2007;82:325–329. doi: 10.1038/sj.clpt.6100283. [DOI] [PubMed] [Google Scholar]
- Higman MA, Port JD, Beauchamp NJ, Jr, Chen AR. Reversible leukoencephalopathy associated with re-infusion of DMSO preserved stem cells. Bone Marrow Transplant. 2000;26:797–800. doi: 10.1038/sj.bmt.1702589. [DOI] [PubMed] [Google Scholar]
- Hijiya N, Hudson MM, Lensing S, Zacher M, Onciu M, Behm FG, Razzouk BI, Ribeiro RC, Rubnitz JE, Sandlund JT, Rivera GK, Evans WE, Relling MV, Pui CH. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA. 2007;297:1207–1215. doi: 10.1001/jama.297.11.1207. [DOI] [PubMed] [Google Scholar]
- Hobson-Webb LD, Roach ES, Donofrio PD. Metronidazole: newly recognized cause of autonomic neuropathy. J Child Neurol. 2006;21:429–431. doi: 10.1177/08830738060210051201. [DOI] [PubMed] [Google Scholar]
- Ingram PR, Howman R, Leahy MF, Dyer JR. Cryptococcal immune reconstitution inflammatory syndrome following alemtuzumab therapy. Clin Infect Dis. 2007;44:e115–117. doi: 10.1086/518168. [DOI] [PubMed] [Google Scholar]
- Isaacson SH, Carr J, Rowan AJ. Ciprofloxacin-induced complex partial status epilepticus manifesting as an acute confusional state. Neurology. 1993;43:1619–1621. doi: 10.1212/wnl.43.8.1619-a. [DOI] [PubMed] [Google Scholar]
- Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS, Kerr H, Stratton P, Duarte RF, McDonald GB, Inamoto Y, Vigorito A, Arai S, Datiles MB, Jacobsohn D, Heller T, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401-e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeljeli M, Guérin‐El Khourouj V, Porcher R, Fahd M, Leveillé S, Yakouben K, Ouachée‐Chardin M, LeGoff J, Cordeiro Debora J, Pédron B, Baruchel A, Dalle JH, Sterkers G. Relationship between cytomegalovirus (CMV) reactivation, CMV-driven immunity, overall immune recovery and graft-versus-leukaemia effect in children. Br J Haematol. 2014;166:229–239. doi: 10.1111/bjh.12875. [DOI] [PubMed] [Google Scholar]
- Jodele S, Dandoy CE, Myers KC, El-Bietar J, Nelson A, Wallace G, Laskin BL. New approaches in the diagnosis, pathophysiology, and treatment of pediatric hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Transfus Apher Sci. 2016;54:181–190. doi: 10.1016/j.transci.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamble RT, Chang CC, Sanchez S, Carrum G. Central nervous system graft-versus-host disease: report of two cases and literature review. Bone Marrow Transplant. 2007;39:49–52. doi: 10.1038/sj.bmt.1705540. [DOI] [PubMed] [Google Scholar]
- Kang JM, Kim YJ, Kim JY, Cho EJ, Lee JH, Lee MH, Lee SH, Sung KW, Koo HH, Yoo KH. Neurologic complications after allogeneic hematopoietic stem cell transplantation in children: analysis of prognostic factors. Biol Blood Marrow Transplant. 2015;21:1091–1098. doi: 10.1016/j.bbmt.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters BK. Central nervous system aspergillosis: a 20-year retrospective series. Hum Pathol. 2002;33:116–124. doi: 10.1053/hupa.2002.30186. [DOI] [PubMed] [Google Scholar]
- Kyllerman M, Himmelmann K, Fasth A, Nordborg C, Månsson JE. Late cerebral graft versus host reaction in a bone marrow transplanted girl with Hurler (MPS I) disease. Neuropediatrics. 2008;39:249–251. doi: 10.1055/s-0028-1112118. [DOI] [PubMed] [Google Scholar]
- Lai PH, Lin SM, Pan HB, Yang CF. Disseminated miliary cerebral candidiasis. AJNR Am J Neuroradiol. 1997;18:1303–1306. [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Yum MS, Kim EH, Kim MJ, Kim KM, Im HJ, Kim YH, Park YS, Ko TS. Clinical characteristics of transplant-associated encephalopathy in children. J Korean Med Sci. 2017;32:457–464. doi: 10.3346/jkms.2017.32.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthra G, Parihar A, Nath K, Jaiswal S, Prasad KN, Husain N, Husain M, Singh S, Behari S, Gupta RK. Comparative evaluation of fungal, tubercular, and pyogenic brain abscesses with conventional and diffusion MR imaging and proton MR spectroscopy. AJNR Am J Neuroradiol. 2007;28:1332–1338. doi: 10.3174/ajnr.A0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Barnes G, Pulliam J, Jezek D, Baumann RJ, Berger JR. CNS angiitis in graft vs host disease. Neurology. 2002;59:1994–1997. doi: 10.1212/01.wnl.0000038948.09158.a7. [DOI] [PubMed] [Google Scholar]
- Maertens J, Theunissen K, Verhoef G, Verschakelen J, Lagrou K, Verbeken E, Wilmer A, Verhaegen J, Boogaerts M, Van Eldere J. Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility study. Clin Infect Dis. 2005;41:1242–1250. doi: 10.1086/496927. [DOI] [PubMed] [Google Scholar]
- Maffini E, Festuccia M, Brunello L, Boccadoro M, Giaccone L, Bruno B. Neurologic complications after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2017;23:388–397. doi: 10.1016/j.bbmt.2016.12.632. [DOI] [PubMed] [Google Scholar]
- Maher OM, Silva JG, Huh WW, Cuglievan B, DePombo A, Kebriaei P, Park M, Liu D, Tillman C, Tarek N, Cooper LJN, Tewari P. Etiologies and impact of readmission rates in the first 180 days after hematopoietic stem cell transplantation in children, adolescents, and young adults. J Pediatr Hematol Oncol. 2017;39:609–613. doi: 10.1097/MPH.0000000000000898. [DOI] [PubMed] [Google Scholar]
- Majhail NS, Brazauskas R, Rizzo JD, Sobecks RM, Wang Z, Horowitz MM, Bolwell B, Wingard JR, Socie G. Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood. 2011;117:316–322. doi: 10.1182/blood-2010-07-294629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino R, Maertens J, Bretagne S, Rovira M, Deconinck E, Ullmann AJ, Held T, Cordonnier C. Toxoplasmosis after hematopoietic stem cell transplantation. Clin Infect Dis. 2000;31:1188–1195. doi: 10.1086/317471. [DOI] [PubMed] [Google Scholar]
- McDonald GB. Hepatobiliary complications of hematopoietic cell transplantation, 40 years on. Hepatology. 2010;51:1450–1460. doi: 10.1002/hep.23533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, Teksam M. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol. 2007;189:904–912. doi: 10.2214/AJR.07.2024. [DOI] [PubMed] [Google Scholar]
- Meadows AT, Friedman DL, Neglia JP, Mertens AC, Donaldson SS, Stovall M, Hammond S, Yasui Y, Inskip PD. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2356–2362. doi: 10.1200/JCO.2008.21.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrmann RL, Mah V, Vinters HV. Neuropathologic findings after bone marrow transplantation: an autopsy study. Hum Pathol. 1990;21:630–639. doi: 10.1016/s0046-8177(96)90010-6. [DOI] [PubMed] [Google Scholar]
- Najima Y, Ohashi K, Miyazawa M, Nakano M, Kobayashi T, Yamashita T, Akiyama H, Sakamaki H. Intracranial hemorrhage following allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2009;84:298–301. doi: 10.1002/ajh.21382. [DOI] [PubMed] [Google Scholar]
- Nguyen MT, Virk IY, Chew L, Villano JL. Extended use dexamethasone-associated posterior reversible encephalopathy syndrome with cisplatin-based chemotherapy. J Clin Neurosci. 2009;16:1688–1690. doi: 10.1016/j.jocn.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Norrby SR. Neurotoxicity of carbapenem antibiotics: consequences for their use in bacterial meningitis. J Antimicrob Chemother. 2000;45:5–7. doi: 10.1093/jac/45.1.5. [DOI] [PubMed] [Google Scholar]
- Ohsaka M, Houkin K, Takigami M, Koyanagi I. Acute necrotizing encephalopathy associated with human herpesvirus-6 infection. Pediatr Neurol. 2006;34:160–163. doi: 10.1016/j.pediatrneurol.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Openshaw H, Stuve O, Antel JP, Nash R, Lund BT, Weiner LP, Kashyap A, McSweeney P, Forman S. Multiple sclerosis flares associated with recombinant granulocyte colony-stimulating factor. Neurology. 2000;54:2147–2150. doi: 10.1212/wnl.54.11.2147. [DOI] [PubMed] [Google Scholar]
- Pana ZD, Seidel D, Skiada A, Groll AH, Petrikkos G, Cornely OA, Roilides E Collaborators of Zygomyco. net and/or FungiScope™ Registries* (2016) Invasive mucormycosis in children: an epidemiologic study in European and non-European countries based on two registries. BMC Infect Dis. 16:667. doi: 10.1186/s12879-016-2005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passweg JR, Baldomero H, Peters C, Gaspar HB, Cesaro S, Dreger P, Duarte RF, Falkenburg JH, Farge-Bancel D, Gennery A, Halter J, Kroger N, Lanza F, Marsh J, Mohty M, Sureda A, Velardi A, Madrigal A European Society for Blood and Marrow Transplantation EBMT. Hematopoietic SCT in Europe: data and trends in 2012 with special consideration of pediatric transplantation. Bone Marrow Transplant. 2014;49:744–750. doi: 10.1038/bmt.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K, Green-Hopkins I, Lu S, Tunkel AR. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis. 2008;12:e111–114. doi: 10.1016/j.ijid.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Pruitt AA, Graus F, Rosenfeld MR. Neurological complications of transplantation: part I: hematopoietic cell transplantation. Neurohospitalist. 2013;3:24–38. doi: 10.1177/1941874412455338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualter E, Satwani P, Ricci A, Jin Z, Geyer MB, Alobeid B, Radhakrishnan K, Bye M, Middlesworth W, Della-Letta P, Behr G, Muniz M, van de Ven C, Harrison L, Morris E, Cairo MS. A comparison of bronchoalveolar lavage versus lung biopsy in pediatric recipients after stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:1229–1237. doi: 10.1016/j.bbmt.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quant E, Wen PY. Neurological complications of hematopoietic stem cell transplantation. In: Schiff D, Kesari S, Wen PY, editors. Cancer Neurology in Clinical Practice, Neurologic Complications of Cancer and Its Treatment. Second Edition. Totawa, NJ, USA: Humana Press; 2008. pp. 327–351. [Google Scholar]
- Rawlinson NJ, Fung B, Gross TG, Termuhlen AM, Skeens M, Garee A, Soni S, Pietryga D, Bajwa RP. Disseminated Rhizomucor pusillus causing early multiorgan failure during hematopoietic stem cell transplantation for severe aplastic anemia. J Pediatr Hematol Oncol. 2011;33:235–237. doi: 10.1097/MPH.0b013e3182050a4f. [DOI] [PubMed] [Google Scholar]
- Rodriguez V, Kuehnle I, Heslop HE, Khan S, Krance RA. Guillain–Barré syndrome after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29:515–517. doi: 10.1038/sj.bmt.1703412. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MR, Pruitt A. Neurologic complications of bone marrow, stem cell, and organ transplantation in patients with cancer. Semin Oncol. 2006;33:352–361. doi: 10.1053/j.seminoncol.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Safdar A, Papadopoulous EB, Armstrong D. Listeriosis in recipients of allogeneic blood and marrow transplantation: thirteen year review of disease characteristics, treatment outcomes and a new association with human cytomegalovirus infection. Bone Marrow Transplant. 2002;29:913–916. doi: 10.1038/sj.bmt.1703562. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber M, Schwender J, Heinz WJ, Zabelina T, Kuhl JS, Mousset S, Schuttrumpf S, Junghanss C, Silling G, Basara N, Neuburger S, Thiel E, Blau IW. Viral encephalitis after allogeneic stem cell transplantation: a rare complication with distinct characteristics of different causative agents. Haematologica. 2011;96:142–149. doi: 10.3324/haematol.2010.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber M, Silling G, Schalk E, Heinz W, Panse J, Penack O, Christopeit M, Buchheidt D, Meyding-Lamadé U, Hähnel S, Wolf HH, Ruhnke M, Schwartz S, Maschmeyer G. CNS infections in patients with hematological disorders (including allogeneic stem-cell transplantation)—Guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO) Ann Oncol. 2016;27:1207–1225. doi: 10.1093/annonc/mdw155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Ruhnke M, Ribaud P, Corey L, Driscoll T, Cornely OA, Schuler U, Lutsar I, Troke P, Thiel E. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood. 2005;106:2641–2645. doi: 10.1182/blood-2005-02-0733. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Marty FM, Holmes TM, Upchurch K, Soiffer RJ, Antin JH, Baden LR, Bromfield EB. Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology. 2007;69:156–165. doi: 10.1212/01.wnl.0000265591.10200.d7. [DOI] [PubMed] [Google Scholar]
- Seishima M, Yamanaka S, Fujisawa T, Tohyama M, Hashimoto K. Reactivation of human herpesvirus (HHV) family members other than HHV-6 in drug-induced hypersensitivity syndrome. Br J Dermatol. 2006;155:344–349. doi: 10.1111/j.1365-2133.2006.07332.x. [DOI] [PubMed] [Google Scholar]
- Servais S, Lengline E, Porcher R, Carmagnat M, Peffault de Latour R, Robin M, Sicre de Fontebrune F, Clave E, Maki G, Granier C, Xhaard A, Dhedin N, Molina JM, Toubert A, Moins-Teisserenc H, Socie G. Long-term immune reconstitution and infection burden after mismatched hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:507–517. doi: 10.1016/j.bbmt.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Sharaf N, Prayson RA. Relapsing polymyositis in chronic graft versus host disease. J Clin Neurosci. 2014;21:1964–1965. doi: 10.1016/j.jocn.2014.03.025. [DOI] [PubMed] [Google Scholar]
- Shkalim-Zemer V, Konen O, Levinsky Y, Michaeli O, Yahel A, Krauss A, Yaniv I, Stein J. Calcineurin inhibitor-free strategies for prophylaxis and treatment of GVHD in children with posterior reversible encephalopathy syndrome after stem cell transplantation. Pediatr Blood Cancer. 2017:64. doi: 10.1002/pbc.26531. [DOI] [PubMed] [Google Scholar]
- Straathof KC, Rao K, Eyrich M, Hale G, Bird P, Berrie E, Brown L, Adams S, Schlegel PG, Goulden N, Gaspar HB, Gennery AR, Landais P, Davies EG, Brenner MK, Veys PA, Amrolia PJ. Haemopoietic stem-cell transplantation with antibody-based minimal-intensity conditioning: a phase 1/2 study. Lancet. 2009;374:912–920. doi: 10.1016/S0140-6736(09)60945-4. [DOI] [PubMed] [Google Scholar]
- Strober J, Cowan MJ, Horn BN. Allogeneic hematopoietic cell transplantation for refractory myasthenia gravis. Arch Neurol. 2009;66:659–661. doi: 10.1001/archneurol.2009.28. [DOI] [PubMed] [Google Scholar]
- Tambasco N, Mastrodicasa E, Salvatori C, Mancini G, Romoli M, Caniglia M, Calabresi P, Verrotti A. Prognostic factors in children with PRES and hematologic diseases. Acta Neurol Scand. 2016;134:474–483. doi: 10.1111/ane.12570. [DOI] [PubMed] [Google Scholar]
- Teive HA, Funke V, Bitencourt MA, de Oliveira MM, Bonfim C, Zanis-Neto J, Medeiros CR, Zetola VF, Werneck LC, Pasquini R. Neurological complications of hematopoietic stem cell transplantation (HSCT): a retrospective study in a HSCT center in Brazil. Arq Neuropsiquiatr. 2008;66:685–690. doi: 10.1590/s0004-282x2008000500014. [DOI] [PubMed] [Google Scholar]
- Tomonari A, Tojo A, Adachi D, Iseki T, Ooi J, Shirafuji N, Tani K, Asano S. Acute disseminated encephalomyelitis (ADEM) after allogeneic bone marrow transplantation for acute myeloid leukemia. Ann Hematol. 2003;82:37–40. doi: 10.1007/s00277-002-0573-1. [DOI] [PubMed] [Google Scholar]
- Tse S, Saunders EF, Silverman E, Vajsar J, Becker L, Meaney B. Myasthenia gravis and polymyositis as manifestations of chronic graft-versus-host-disease. Bone Marrow Transplant. 1999;23:397–399. doi: 10.1038/sj.bmt.1701575. [DOI] [PubMed] [Google Scholar]
- Tsutsumi Y, Kanamori H, Kawamura T, Umehara S, Obara S, Ogura N, Shimoyama N, Tanaka J, Asaka M, Imamura M, Masauzi N. Inflammatory pseudotumor of the brain following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35:1123–1124. doi: 10.1038/sj.bmt.1704955. [DOI] [PubMed] [Google Scholar]
- Uckan D, Cetin M, Yigitkanli I, Tezcan I, Tuncer M, Karasimav D, Oguz KK, Topcu M. Life-threatening neurological complications after bone marrow transplantation in children. Bone Marrow Transplant. 2005;35:71–76. doi: 10.1038/sj.bmt.1704749. [DOI] [PubMed] [Google Scholar]
- Unal S, Sag E, Kuskonmaz B, Kesici S, Bayrakci B, Ayvaz DC, Tezcan I, Yalnizoglu D, Uckan D. Successful treatment of severe myasthenia gravis developed after allogeneic hematopoietic stem cell transplantation with plasma exchange and rituximab. Pediatr Blood Cancer. 2014;61:928–930. doi: 10.1002/pbc.24799. [DOI] [PubMed] [Google Scholar]
- Uy GL, Costa LJ, Hari PN, Zhang MJ, Huang JX, Anderson KC, Bredeson CN, Callander NS, Cornell RF, Perez MA, Dispenzieri A, Freytes CO, Gale RP, Garfall A, Gertz MA, Gibson J, Hamadani M, Lazarus HM, Kalaycio ME, Kamble RT, et al. Contribution of chemotherapy mobilization to disease control in multiple myeloma treated with autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2015;50:1513–1518. doi: 10.1038/bmt.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdeguer A, de Heredia CD, González M, Martínez AM, Fernández-Navarro JM, Pérez-Hurtado JM, Badell I, Gómez P, González ME, Muñoz A, Díaz MA GETMON: Spanish Working Party for Blood and Marrow Transplantation in Children. Observational prospective study of viral infections in children undergoing allogeneic hematopoietic cell transplantation: a 3-year GETMON experience. Bone Marrow Transplant. 2010;46:119. doi: 10.1038/bmt.2010.52. [DOI] [PubMed] [Google Scholar]
- Viscoli C, Garaventa A, Ferrea G, Manno G, Taccone A, Terragna A. Listeria monocytogenes brain abscesses in a girl with acute lymphoblastic leukaemia after late central nervous system relapse. Eur J Cancer. 1991;27:435–437. doi: 10.1016/0277-5379(91)90380-v. [DOI] [PubMed] [Google Scholar]
- Walter AW, Hancock ML, Pui CH, Hudson MM, Ochs JS, Rivera GK, Pratt CB, Boyett JM, Kun LE. Secondary brain tumors in children treated for acute lymphoblastic leukemia at St Jude Children's Research Hospital. J Clin Oncol. 1998;16:3761–3767. doi: 10.1200/JCO.1998.16.12.3761. [DOI] [PubMed] [Google Scholar]
- Wong FL, Bosworth A, Danao R, Villaluna D, Patel S, Grant M, Forman SJ, Bhatia S. Neurocognitive function and its impact on return to work in patients treated with hematopoietic cell transplantation (HCT) Blood. 2009;114:216–216. [Google Scholar]
- Yamada K, Shrier DA, Rubio A, Shan Y, Zoarski GH, Yoshiura T, Iwanaga S, Nishimura T, Numaguchi Y. Imaging findings in intracranial aspergillosis. Acad Radiol. 2002;9:163–171. doi: 10.1016/s1076-6332(03)80166-6. [DOI] [PubMed] [Google Scholar]
- Ye B, Yu D, Zhang X, Shao K, Chen D, Wu D, Zhang Y, Zhou Y, Shen Y, Yu Q. Disseminated Rhizopus microsporus infection following allogeneic hematopoietic stem cell transplantation in a child with severe aplastic anemia. Transpl Infect Dis. 2013;15:E216–223. doi: 10.1111/tid.12144. [DOI] [PubMed] [Google Scholar]
- Yuan C, Deberardinis C, Patel R, Shroff SM, Messina SA, Goldstein S, Mori S. Progressive multifocal leukoencephalopathy after allogeneic stem cell transplantation: Case report and review of the literature. 2018:e12879. doi: 10.1111/tid.12879. [DOI] [PubMed] [Google Scholar]
- Zama D, Gasperini P, Berger M, Petris M, De Pasquale MD, Cesaro S, Guerzoni ME, Mastrodicasa E, Savina F, Ziino O, Kiren V, Muggeo P, Mura RM, Melchionda F, Zanazzo GA Supportive Therapy Working Group of Italian Pediatric Haematology and Oncology Association (AIEOP) A survey on hematology-oncology pediatric AIEOP centres: The challenge of posterior reversible encephalopathy syndrome. Eur J Haematol. 2018;100:75–82. doi: 10.1111/ejh.12984. [DOI] [PubMed] [Google Scholar]
- Zaoutis TE, Heydon K, Chu JH, Walsh TJ, Steinbach WJ. Epidemiology, outcomes, and costs of invasive aspergillosis in immunocompromised children in the United States 2000. Pediatrics. 2006;117:e711–716. doi: 10.1542/peds.2005-1161. [DOI] [PubMed] [Google Scholar]
- Zekri AR, Mohamed WS, Samra MA, Sherif GM, El-Shehaby AM, El-Sayed MH. Risk factors for cytomegalovirus, hepatitis B and C virus reactivation after bone marrow transplantation. Transpl Immunol. 2004;13:305–311. doi: 10.1016/j.trim.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Zhang XH, Wang QM, Chen H, Chen YH, Han W, Wang FR, Wang JZ, Zhang YY, Mo XD, Chen Y, Wang Y, Chang YJ, Xu LP, Liu KY, Huang XJ. Clinical characteristics and risk factors of Intracranial hemorrhage in patients following allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2016;95:1637–1643. doi: 10.1007/s00277-016-2767-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neurological complications of hematopoietic cell transplantation.