Abstract
Background
Many clinicians and insurance providers are reluctant to embrace recent guidelines identifying people who inject drugs (PWID) as a priority population to receive hepatitis C virus (HCV) treatment. The aim of this study was to evaluate the efficacy of direct-acting antiviral (DAA) HCV therapy in a real-world population comprised predominantly of PWID.
Methods
A retrospective analysis was performed on all HCV-infected patients who were treated at the Vancouver Infectious Diseases Centre between March 2014 and December 2017. All subjects were enrolled in a multidisciplinary model of care, addressing medical, psychological, social, and addiction-related needs. The primary outcome was achievement of sustained virologic response (undetectable HCV RNA) 12 or more weeks after completion of HCV therapy (SVR-12).
Results
Overall, 291 individuals were enrolled and received interferon-free DAA HCV therapy. The mean age was 54 years, 88% were PWID, and 20% were HCV treatment experienced. At data lock, 62 individuals were still on treatment and 229 were eligible for evaluation of SVR by intent-to-treat (ITT) analysis. Overall, 207 individuals achieved SVR (90%), with 13 losses to follow-up, 7 relapses, and 2 premature treatment discontinuations. ITT SVR analysis show that active PWID and treatment-naïve patients were less likely to achieve SVR (P = .0185 and .0317, respectively). Modified ITT analysis of active PWID showed no difference in achieving SVR (P = .1157) compared with non-PWID.
Conclusion
Within a multidisciplinary model of care, the treatment of HCV-infected PWID with all-oral DAA regimens is safe and highly effective. These data justify targeted efforts to enhance access to HCV treatment in this vulnerable and marginalized population.
Keywords: hepatitis C virus, interferon-free direct-acting antiviral therapy, multidisciplinary, PWID
The global burden of hepatitis C virus (HCV) infection continues to rise, with core-transmitters such as people who inject drugs (PWID) making up the majority of incident (80%) and prevalent (60%) cases [1, 2]. Despite these facts, HCV treatment programs for PWID have not yet been a major part of the response to the pandemic, with less than 10% of infected individuals belonging to this group having received HCV therapy [3].
Historically, treatment consisted of injected interferon and oral ribavirin (RBV), with modest sustained virologic response (SVR) rates of 40%–50% and significant adverse effects such as anemia, flu-like symptoms, and psychiatric symptoms [4]. The advent of interferon-free direct-acting antiviral (DAA) therapies has transformed HCV into an easily curable disease, with SVR rates as high as 95% [5, 6]. DAA regimens that are highly efficacious, well-tolerated, and relatively short in duration are now available for all HCV genotypes and for patient populations historically considered difficult to cure [7]. The ease of dosing, safety profile, and effectiveness of these agents provide an opportunity to expand the number of patients who can be treated for HCV [8]. In clinical trials involving PWID populations, these therapies are equally effective [9]. However, many providers remain reluctant to offer HCV therapy to PWID, citing concerns around treatment adherence, poorer outcomes compared with people who do not inject drugs, and the subsequent risk of HCV reinfection [10, 11]. Post-treatment loss to follow-up (LTFU) in this population can be as high as 13% in some situations, highlighting another key challenge in this group [12].
It has been shown that multidisciplinary models of care are effective in treating HCV and other health and social issues in the PWID population, with relatively simple programs that can be established to engage PWID in care [13]. Furthermore, a model that successfully engages PWID in care can lead to high treatment uptake and reduction in the rate of reinfection [14]. Although clinical trials using all currently available interferon-free DAA regimens confirm high SVR rates, treatment barriers in the real world may play an important role in achieving HCV elimination by 2030 [15, 16]. It is often stated that clinical trials produce higher cure rates than what may be expected in practice given increased population heterogeneity and the lack of visit incentivization in the real world [17]. It is therefore critical to confirm the efficacy of DAAs in a real-world setting, especially in marginalized populations such as PWID, while ensuring that loss to follow-up (LTFU) is minimized and monitoring is in place to assess for re-infection. The aim of this analysis is to evaluate the safety and efficacy of currently available interferon-free DAA regimens for the treatment of HCV in a real-world population, consisting mostly of PWID.
METHODS
Setting and Design
In this retrospective cohort study, we extracted and analyzed data from a common database for all HCV-infected patients in our clinic, the Vancouver Infectious Diseases Centre (VIDC). Specifically, we included all patients documented to have started hepatitis C treatment between March 2014 and December 2017. Patients included in this study had to fulfill 2 main criteria: a positive test for HCV RNA at any point during their lifetime and initiation of a partial or complete course of HCV therapy. Patients were initiated on antiviral therapy based on a medical indication to do so: chronic detectable viremia and expectation of engagement in care for the duration of the proposed therapy. Patient willingness to receive treatment was evaluated qualitatively through consultation with infectious diseases specialists and on-site nurses. There were no specific age, ethnicity, or gender-related inclusion criteria. Individuals were classified as PWID if they had any history of injection drug use, and recent PWID if they had injected drugs in the last 6 months (verified by a urine drug screen and physician consultation). Regardless of drug use status, all HCV viremic patients were offered treatment. The only case in which patients did not receive treatment was when patients refused HCV treatment. At the time of data lock (December 2017), HCV treatments were reimbursed in British Columbia if patients presented with fibrosis scores of F2-F4 and were given conditional access if comorbidities such as HIV, hepatitis B virus (HBV), chronic kidney disease, or other extrahepatic manifestations were present. Available treatments included elbasvir/grazoprevir, sofosbuvir/velpatasvir, and sofosbuvir/ledipsvir. In a situation where government reimbursement was not possible, DAAs were acquired through private compassionate care programs or through private insurers. In general, all patients treated at our center did not need to pay out of pocket for HCV treatment.
The Multidisciplinary Model and LTFU Management
All patients at the VIDC had access to comprehensive multidisciplinary care with nursing, medical care (including infectious diseases specialty care), and logistic support, which we refer to as the “4-legged chair” model. This model addresses psychiatric, addiction-related, social, and medical needs in an integrated manner. Infectious diseases specialists managed patients’ medical, addiction, and psychiatric needs, apart from methadone prescription, which requires additional licensing in the province of British Columbia (patients requiring methadone were referred to partner physicians that work closely with our center to maintain patients in care). Nurses and clinic staff assisted with any government forms patients needed help with. Patients were offered these services regardless of whether they were on HCV treatment. Patients had access to weekly educational support groups, as well as nutritional assistance and access to nonprescription medications at no charge. The support groups provide the ability to identify specific medical or social needs and have them addressed through personalized interactions with clinic staff. Before initiating treatment, patients were educated on HCV and their chosen regimen, and during their very first visit to our center, they received a FibroScan. Receiving a FibroScan during the first visit encourages engagement as patients feel that they are already on the way to receiving treatment. During treatment, patients were well integrated into our model of care and were provided with assistance with forms and paperwork for social services such as housing, income assistance, and nutritional support. Post-SVR, patients were followed up through standard of care, and attempts were made to follow patients up every 6 months to monitor HCV reinfection and other comorbidities. Active injection drug users were followed-up every 3 months or as needed. If a patient was suspected to be LTFU, steps were taken to re-engage the patient in care and obtain SVR12 bloodwork, or to re-initiate treatment. These steps included contacting patients’ pharmacies, opiate substitution therapy (OST) providers, primary care physicians, and shelters. Once patients had been contacted, they were encouraged to attend VIDC and re-engage in care. On occasion, patients who had previously been LTFU were encountered at outreach events, and attempts were made to re-engage them in care. In this analysis, a patient is defined as LTFU if all avenues of engagement were exhausted and the patient did not attend VIDC within 6 months of their SVR12 time point.
Study Variables
We obtained patients’ demographic characteristics, including age and sex. Clinical and laboratory data included previous HCV treatment, liver fibrosis at baseline, baseline HCV RNA level, coinfection with HIV, HCV genotype, OST status, drug use, and other social and demographic variables. Stage of liver fibrosis was assessed by liver stiffness measurement (FibroScan). For liver stiffness measurements, the chosen cutoffs for significant liver fibrosis and cirrhosis were 7.1 kPa and 12.5 kPa, respectively.
Outcomes
The primary outcome of interest was undetectable virus (<12 IU/mL) 12 weeks after the end of therapy (SVR). We also assessed LTFU before the end of treatment and before the SVR time point.
Statistical Analysis
The intent-to-treat (ITT) outcome was SVR and included all patients. The modified intent-to-treat (mITT) outcome excluded patients who were LTFU after completing treatment, and per-protocol analysis excluded all LTFU and treatment discontinuations to evaluate the pure efficacy of DAAs. Treatment discontinuation was defined as taking minimum 1 tablet but not completing the planned treatment course. A sample size calculation was not done because our intention was to capture all individuals who initiated HCV DAA therapy between March 2014 and December 2017. Bivariate analysis was done using the Fisher exact test for categorical data, and confidence intervals for proportions were expressed with 95% confidence using the Clopper-Pearson method. For all analyses, we used 2-sided P values of .05 as the cutoff for statistically significant differences. All data management and analyses were performed using Software Package for the Social Sciences 25.0 (SPSS).
Ethics Approval
This study and patient data collection were approved by Chesapeake Institutional Review Board (IRB) Ethics Board and were annually renewed as required. Procedures followed were in accordance with the ethical standards of the Chesapeake IRB and the Helsinki Declaration. Patient’s written consent was obtained, and any identifying information was anonymized.
RESULTS
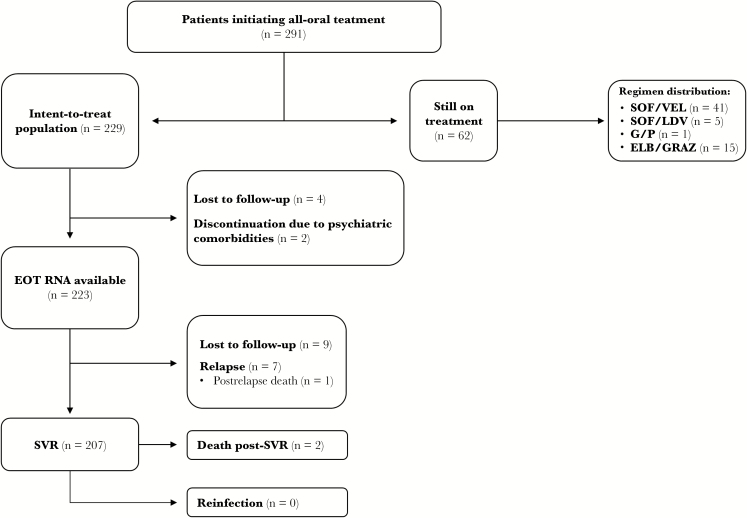
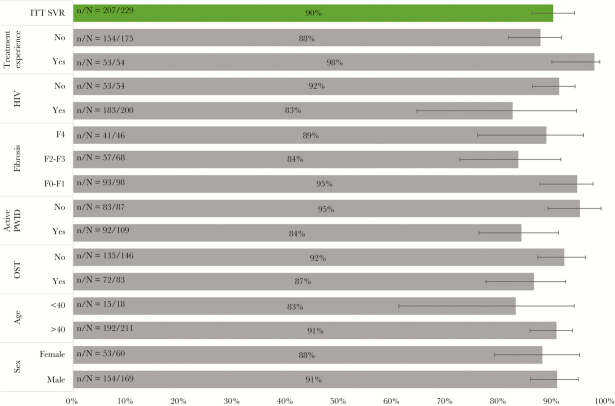
A total of 291 individuals initiated interferon-free DAA HCV therapy at the VIDC between May 1, 2014, and December 31, 2017. The demographics of the cohort are outlined in Table 1. Notably, in this patient population, 256 (88%) were drug users, 134 (46%) had actively used drugs in the past 6 months, 105 (36%) were receiving opiate substitution therapy (OST), and 36 (13%) presented with HIV co-infection. The most prevalent genotype was 1a, at 63%, and 64 (22%) individuals were assessed to have cirrhosis at baseline evaluation. With respect to individuals actively injecting drugs (n = 134), 110 (82%) injected opiates, 60 (45%) injected cocaine and amphetamines, and 31 (24%) injected other/unknown substances (documented by urine drug screen). As of data lock (December 31, 2017), 229 individuals were eligible for ITT analysis, whereas 62 patients were still on treatment. Among those still on treatment, 45 were on sofosbuvir (SOF)-based regimens, 1 was on glecaprevir/pibrentasvir, and 15 were on elbasvir (ELB)/grazoprevir (GRAZ). Between treatment initiation and the end of treatment (EOT), 4 individuals were suspected to be LTFU, and 2 patients discontinued treatment due to psychiatric comorbidities. Between the EOT and SVR time points, 9 individuals were suspected to be LTFU and 7 experienced viral relapses (there was 1 death post–HCV relapse unrelated to HCV treatment). Overall, 207 individuals achieved SVR, with an ITT SVR rate of 90%, an mITT SVR rate of 93%, and a per-protocol SVR rate of 96%. There were 2 deaths post-SVR, both due to unrelated extrahepatic comorbidities. There were no confirmed cases of reinfection via deep sequencing of genotypes (Figure 1; Table 2). The range of follow-up post-SVR (median) was 1–155 (80) weeks. The prevalence of LTFU was 6% (n/N = 13/229). ITT SVR rates based on specific regimens ranged from 87% to 94%. Of 7 relapses, 3 were with paritaprevir/ritonavir/ombitasvir plus dasabuvir (PRoD), 3 with sofosbuvir/ledipasvir (SOF/LDV), and 1 with sofosbuvir/velpatisvir (SOF/VEL) (Table 2). Comparison of HCV regimens showed no significant difference in ITT SVR rates (Table 3). Stratified ITT SVR rates by key variables show that active PWID were significantly less likely to achieve SVR (P = .0185), and individuals with previous HCV treatment experience were significantly more likely to achieve SVR (P = .0317). Analysis showed that sex, age, OST status, fibrosis level, HIV status, and drug use during treatment were not significant factors in achieving SVR (Figure 2; Table 4). Further analysis into the active PWID cohort showed that those who injected in the past 6 months achieved an SVR rate of 84%. Individuals in this cohort made up 70% of all observed LTFU, 100% of all discontinuations, and 86% of all relapses. Of 9 LTFU among active PWID, 4 occurred pre-EOT and 5 post-EOT (all post-EOT LTFU had an undetectable viral load at EOT). An mITT analysis gives an SVR rate of 88% in this cohort, and there was no significant difference in cure rates between mITT rates of active and non-active PWID (P = .1157) (Table 5).
Table 1.
Demographics of Total Cohort on Interferon-Free DAA Therapy
| Baseline Characteristics | All-Oral Therapy |
|---|---|
| Patients, n | 291 |
| Mean age (SD), y | 54 (10) |
| Female sex, n (%) | 78 (27) |
| Homeless, n (%) | 36 (12) |
| Method of engagement, n (%) | 200 (64) |
| Referral from another physician | 68 (23) |
| Community pop-up clinics | 5 (2) |
| Prison | 18 (7) |
| No data | |
| PWID, n (%) | 256 (88) |
| Active drug use (past 6 mo), n (%) | 134 (46) |
| Opiates | 110 (82) |
| Cocaine | 60 (45) |
| Amphetamines | 60 (45) |
| Other | 22 (17) |
| Unknown | 9 (7) |
| Active drug use during treatment, n (%) | 90 (31) |
| Any alcohol use, n (%) | 86 (29) |
| OST prescription, n (%) | 105 (36) |
| Methadone | 84 (80) |
| Buprenorphine/naloxone | 11 (10) |
| Other | 10 (10) |
| HIV co-infection, n (%) | 36 (13) |
| HCV genotype, n (%) | 183 (63) |
| 1a | 22 (8) |
| 1b | 19 (7) |
| 2 | 65 (22) |
| 3 | 2 (1) |
| 4 | |
| Stage of liver disease, n (%) | |
| No or mild fibrosis (F0-F1) | 124 (42) |
| Mild or advanced fibrosis (F2-F3) | 85 (29) |
| Cirrhosis (F4) | 64 (22) |
| No data | 18 (7) |
| HCV treatment experienced, n (%) | 59 (20) |
Abbreviations: DAA, direct-acting antiviral; HCV, hepatitis C virus; OST, opiate substitution therapy; PWID, people who inject drugs.
Figure 1. .
Flow diagram showing patients with available data and various outcomes.
Table 2.
Overall SVR Rate and SVR Rates Per Regimen
| Completed Regimens and Available SVR12 | Value | HCV Cure | ITT SVR, % | 95% CI | mITT SVR, % | Per-Protocol SVR, % | Relapse | LTFU/DC |
|---|---|---|---|---|---|---|---|---|
| Overall | 229 | 207 | 90 | 86–94 | 93 | 96 | 7 | 13/2 |
| PRoD | 80 | 73 | 88 | 83–96 | 95 | 96 | 3 | 3/1 |
| ELB/GRAZ | 31 | 27 | 87 | 71–95 | 95 | 100 | 0 | 4/0 |
| SOF/LDV | 58 | 51 | 88 | 77–94 | 91 | 94 | 3 | 4/0 |
| SOF/VEL based | 32 | 30 | 94 | 80–98 | 94 | 97 | 1 | 0/1 |
| Other regimens | 28 | 26 | 93 | 77–98 | 93 | 100 | 0 | 2/0 |
| RBV based | 96 | 88 | 92 | 84–96 | 94 | 97 | 3 | 4/1 |
Abbreviations: DC, discontinuation; ELB/GRAZ, elbasvir/grazoprevir; ITT, intent-to-treat; LDV, ledispasvir; LTF, lost to follow-up; mITT, modified intent-to-treat; PRoD, paritaprevir/ritonavir/ombitasvir plus dasabuvir; RBV, ribavirin; SOF, sofosbuvir; SVR, sustained virologic response; VEL, velpatisvir.
Table 3.
Comparison of HCV Regimens
| Regimen | Compared With: | P Value |
|---|---|---|
| PRoD | ELB/GRAZ | .4968 |
| SOF/LDV | .5756 | |
| SOF/VEL based | 1.000 | |
| Other regimens | 1.000 | |
| ELB/GRAZ | SOF/LDV | 1.000 |
| SOF/VEL based | .4258 | |
| Other regimens | .6732 | |
| SOF/LDV | SOF/VEL based | .7133 |
| Other regimens | .7187 | |
| SOF/VEL | Other regimens | 1.000 |
Abbreviations: ELB/GRAZ, elbasvir/grazoprevir; LDV, ledispasvir; PRoD, paritaprevir/ritonavir/ombitasvir plus dasabuvir; SOF, sofosbuvir; VEL, velpatisvir.
Figure 2.
Stratified intent-to-treat sustained virologic response rates by key variables.
Table 4.
Significance of ITT SVR Rates by Key Variables
| Variable | P Value (ITT Analysis) | |
|---|---|---|
| Sex | Male | .4426 |
| Age | >40 | .3928 |
| <40 | ||
| OST | Yes | .1685 |
| No | ||
| Active PWID | Yes | .0185 |
| No | ||
| Fibrosis | F0-F1 | .061421 |
| F2-F3 | ||
| F4 | ||
| HIV | Yes | .3022 |
| No | ||
| Treatment experience | Yes | .0317 |
| No | ||
| Drug use during treatment | Yes | .8182 |
| No | ||
Abbreviations: active PWID, injected drugs in past 6 months; ITT, intent-to-treat; OST, opiate substitution therapy; PWID, people who inject drugs.
Table 5.
Active PWID Subanalysis
| Active PWID (n = 109) | Value |
|---|---|
| SVR, n (%) | 92 (84) |
| Non-SVR, n (%) | 17 (16) |
| Reason for non-SVR | |
| LTFU (% of all LTFU) | 9 (70) |
| D/C (% of all D/C) | 2 (100) |
| Relapse (% of all relapse) | 6 (86) |
| LTFU in active PWID (n = 9) | |
| Pre-EOT LTFU, n (%) | 4 (44) |
| Post-EOT LTFU, n (%) | 5 (66) |
| Undetectable HCV RNA at EOT, n (%) | 5 (100) |
| mITT SVR in active PWID, % | 88 |
| P value (vs mITT in nonactive PWID) | .1157 |
| Injection during treatment, n (%) | 8 (89) |
Abbreviations: active PWID, injected drugs in past 6 months; D/C, discontinuation; EOT, end of treatment; ITT, intent-to-treat; LTFU, lost to follow-up; mITT, modified intent-to-treat; OST, opiate substitution therapy; PWID, people who inject drugs; SVR, sustained virological response.
DISCUSSION
This study presents real-world data on the efficacy of interferon-free DAA HCV therapy in a vulnerable population, 88% of whom were identified as PWID. With an overall ITT SVR rate of 90%, an mITT SVR rate of 93%, and a low rate of LTFU of 6%, these data provide evidence to support expansion of HCV treatment programs among PWID, as recommended by the most current guideline documents [18, 19].
These data reproduce the SVR rates reported in post hoc analyses of patients enrolled in the registrational studies of the DAA regimens that we utilized [20, 21]. They reproduce the SVR rates (90%–95%) obtained in the only preregistrational study allowing participation of active PWID (C-EDGE CO-STAR) and the SVR rate [22]. They reproduce the SVR rate (94%) of the SIMPLIFY study of sofosbuvir/velpatasvir, conducted exclusively among recent or current PWID [23, 24]. And, importantly, the rigorous patient retention strategies we have employed have produced LTFU rates lower than those reported in real-world cohorts of non-PWID populations. Anecdotally, since the data lock that preceded this analysis, 9/13 LTFU individuals have been re-engaged in care, producing a residual LTFU rate of 2% (n/N = 4/229). This suggests that a multidisciplinary approach to managing social, psychiatric, medical, and addiction-related needs could be a highly relevant strategy to engage patients in care and re-engage those who were LTFU [25]. In fact, the use of multidisciplinary systems is key to addressing addiction-related needs, as treatment o f PWID in nonmultidisciplinary settings is not recommended given the diversity of their needs [26]. Of 3 deaths (all occurring post-EOT), 1 was due to an opiate overdose, highlighting the high-risk nature of this population and the critical need for enhanced post-treatment follow-up.
To our knowledge, there have been no real-world studies comparing the efficacy of interferon-free DAA therapies in the PWID population. Given the size of our cohort, we chose to compare, in an exploratory fashion, the SVR rates obtained with PRoD, ELB/GRAZ, SOF/LDV, and SOF/VEL, and we did not detect any significant differences in the efficacy of these regimens. Del Rio-Valencia et al. found a significant difference in SVR rates comparing SOF/LDV and SOF/daclatasvir (DCV), with SOF/DCV proving to be more effective in genotype 3 patients [27]. However, using SOF/LDV in patients with genotype 3 is an off-label utilization, hence the low SVR rates [18]. This suggests that when treating HCV infection among PWID, health care providers should feel comfortable prescribing any of the available DAA regimens as long as they are being used according to label indications.
Stratified SVR rates by key variables and comparison of these variables show that treatment-experienced patients were significantly (P = .0317) more likely to achieve SVR. In most cases, the prior regimens included interferon, and most cases were virologic relapses. This population may have an enhanced response to subsequent therapy, from a biologic perspective [28, 29]. There may also be a motivational component to succeed in the setting of prior failure of a regimen with major adverse events [4]. This may have enhanced commitment to the current course of treatment and enhanced adherence, leading to the slightly higher SVR rates we observed.
More active PWID (those injecting in the past 6 months) were also significantly less likely to achieve SVR compared with less active PWID (P = .0185). This did not relate to virologic failure, but LTFU. No significant difference between the 2 groups was observed on mITT analysis (P = .1157). This highlights the critical need to address LTFU in populations such as these, particularly those who are injecting drugs during treatment, as 89% of those who were LTFU belonged to this subgroup. It is worth noting that those who were LTFU post-EOT all had undetectable viral loads, confirming cure and demonstrating the efficacy of DAA therapy even in the setting of active drug use during treatment. This can be achieved when multidisciplinary systems are implemented and active approaches to minimize LTFU are in place.
This study has some limitations. Patients were not randomly selected to receive HCV treatment or to be integrated into our multidisciplinary model of care. It is possible that the patients on whom we report were more highly motivated to receive treatment and that they are not completely representative of the PWID population as a whole. Conversely, due to fear of stigmatization, it may be that recreational drug use was under-reported in some cases and that some patients were misclassified as non-recent PWID. If this is the case, the significance of our results would be further enhanced. Finally, this is a single-arm, noncomparative analysis. It is not possible to evaluate which of the components of our model contributed directly to its success. It could be worthwhile to conduct a randomized study to address this issue to inform public health policy around more widespread implementation of HCV treatment programs in this population.
In conclusion, HCV treatment delivered to PWID within a multidisciplinary program of care to address medical, social, psychological, and addiction-related aspects of care leads to very high SVR rates and very low rates of LTFU, irrespective of the DAA regimen prescribed. Our findings add to the body of knowledge supporting the expansion of HCV treatment programs in this vulnerable population, which includes a high number of core-transmitters of the infection. Approaches such as ours will be essential to the achievement of the goals of the World Health Organization to eliminate HCV as a public health concern by 2030 [16].
Acknowledgments
Author contributions. B.C., D.T., and A.A. conceived of the project. A.A. and J.H. did the literature searches and retrospective chart review. A.A. and A.T. conducted the statistical analysis. A.A. and B.C. drafted the manuscript, and all authors contributed to its final version. All authors have seen and approved the final version.
We thank the Vancouver Infectious Diseases Centre (VIDC) patients and staff for their contributions to this work. We would also like to thank our funders, the Canadian Institutes of Health Research, CanHepC, Merck & Co, Gilead Sciences, AbbVie, and ViiV Healthcare.
Financial support. The VIDC is partly supported by the Canadian Institutes of Health Research, CanHepC, Merck & Co, Gilead Sciences, AbbVie, and ViiV Healthcare.
Potential conflicts of interest. A.A. reports travel grants from AbbVie and Merck & Co. J.H. and A.T. have nothing to declare. D.T. reports honoraria from Merck & Co. B.C. reports grants, honoraria, travel funding, and advisory board positions with AbbVie, Merck & Co, Gilead Sciences, and ViiV. This analysis was conducted as part of the VIDC’s overall research program, and no funding was specifically dedicated to it.
References
- 1. Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017; 2:161–76. [DOI] [PubMed] [Google Scholar]
- 2. Degenhardt L, Peacock A, Colledge S, et al. . Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 2017; 5:e1192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alavi M, Raffa JD, Deans GD, et al. . Continued low uptake of treatment for hepatitis C virus infection in a large community-based cohort of inner city residents. Liver Int 2014; 34:1198–206. [DOI] [PubMed] [Google Scholar]
- 4. Bennett JE. Infectious diseases and their etiological agents. In: John E, Dolin R, Blaser MJ, Douglas RG, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed Philadelphia, PA: Elsevier/Saunders; 2015; 34:2157–2187. [Google Scholar]
- 5. Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. JAMA 2014; 312:631–40. [DOI] [PubMed] [Google Scholar]
- 6. Grebely J, Hajarizadeh B, Dore GJ. Direct-acting antiviral agents for HCV infection affecting people who inject drugs. Nat Rev Gastroenterol Hepatol 2017; 14(11):641–51. [DOI] [PubMed] [Google Scholar]
- 7. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, et al. . Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med 2017; 166:637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. Testing for HCV infection: an update of guidance for clinicians and laboratories. MMWR Morb Mortal Wkly Rep 2013; 62:362–5. [PMC free article] [PubMed] [Google Scholar]
- 9. Grebely J, Litwin A, Dore GJ. Addressing reimbursement disparities for direct-acting antiviral therapies for hepatitis C virus infection is essential to ensure access for all. J Viral Hepat 2016; 23:664–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barua S, Greenwald R, Grebely J, et al. . Restrictions for medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med 2015; 163:215–23. [DOI] [PubMed] [Google Scholar]
- 11. Marshall AD, Nielsen S, Cunningham EB, et al. . Restrictions for reimbursement of interferon-free direct-acting antiviral therapies for HCV infection in Europe. J Hepatol 2017; 66:S95–96. [DOI] [PubMed] [Google Scholar]
- 12. Read P, Lothian R, Chronister K, et al. . Delivering direct acting antiviral therapy for hepatitis C to highly marginalised and current drug injecting populations in a targeted primary health care setting. Int J Drug Policy 2017; 47:209–15. [DOI] [PubMed] [Google Scholar]
- 13. Grebely J, Genoway K, Khara M, et al. . Treatment uptake and outcomes among current and former injection drug users receiving directly observed therapy within a multidisciplinary group model for the treatment of hepatitis C virus infection. Int J Drug Policy 2007; 18:437–43. [DOI] [PubMed] [Google Scholar]
- 14. Alimohammadi A, Singh A, Raycraft T, et al. . Reduced HCV recurrent viremia in people who inject drugs (PWID) after treatment induced HCV cure. Int J Adv Res 2017; 6:2075–7. [Google Scholar]
- 15. Bansal S, Singal AK, McGuire BM, Anand BS. Impact of all oral anti-hepatitis C virus therapy: a meta-analysis. World J Hepatol 2015; 7:806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The Lancet. Towards elimination of viral hepatitis by 2030. Lancet 2016; 388(10042):308. [DOI] [PubMed] [Google Scholar]
- 17. Zoulim F, Liang TJ, Gerbes AL, et al. . Hepatitis C virus treatment in the real world: optimising treatment and access to therapies. Gut 2015; 64:1824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. EASL. EASL recommendations on treatment of hepatitis C 2016. J Hepatol 2017; 66:153–94. [DOI] [PubMed] [Google Scholar]
- 19. AASLD. Recommendations for testing, managing, and treating hepatitis C. 2016. Available at: http://hcvguidelines.org/sites/default/files/HCV-Guidance_July_2016_b.pdf. Accessed 18 Febuary 2018.
- 20. Aspinall AI, Shaheen AA, Kochaksaraei GS, et al. . Real-world treatment of hepatitis C with second-generation direct-acting antivirals: initial results from a multicentre Canadian retrospective cohort of diverse patients. CMAJ Open 2018; 6:E12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dore G, Altice F, Litwin AH, et al. . C-edge co-star: efficacy of grazoprevir and elbasvir in persons who inject drugs (PWID) receiving opioid agonist therapy. Hepatology 2015; 62(suppl):227A–8A. [Google Scholar]
- 22. Dore GJ, Grebely J, Altice F, et al. . Hepatitis C virus (HCV reinfection and injecting risk behavior following elbasvir (EBR)/grazoprevir (GZR) treatment in participants on opiate agonist therapy (OAT): CO-STAR part B. Hepatology 2017; 66:112a–13a. [Google Scholar]
- 23. Terrault NA, Zeuzem S, Di Bisceglie AM, et al. ; HCV-TARGET Study Group Effectiveness of ledipasvir-sofosbuvir combination in patients with hepatitis C virus infection and factors associated with sustained virologic response. Gastroenterology 2016; 151:1131–1140.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grebely J, Dalgard O, Conway B, et al. . Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. In press. [DOI] [PubMed] [Google Scholar]
- 25. Wohl DA, Allmon AG, Evon D, et al. . Financial incentives for adherence to hepatitis C virus clinical care and treatment: a randomized trial of two strategies. Open Forum Infect Dis 2017; 4:ofx095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robaeys G, Grebely J, Mauss S, et al. . Recommendations for the management of hepatitis C virus infection among people who inject drugs. Clin Infect Dis 2013; 57(Suppl 2):S129–37. [DOI] [PubMed] [Google Scholar]
- 27. Del Rio-Valencia JC, Asensi-Diez R, Madera-Pajin R, et al. . Interferon-free treatments in patients with hepatitis C genotype 3 infection in a tertiary hospital. Rev Esp Quimioter 2018; 31:35–42. [PMC free article] [PubMed] [Google Scholar]
- 28. Ge D, Fellay J, Thompson AJ, et al. . Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009; 461:399–401. [DOI] [PubMed] [Google Scholar]
- 29. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. . Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 2014; 370:211–21. [DOI] [PubMed] [Google Scholar]