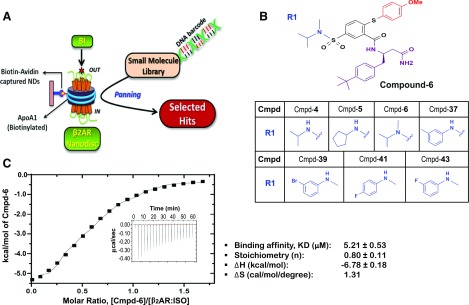
Fig. 1.
Hit compounds from DEL screening with the agonist-occupied β2AR in HDL particles. (A) Cartoon for DEL screening. Purified human β2ARs were reconstituted in HDL particles (β2AR Nanodiscs) and then occupied by BI-167107 (BI). DNA-encoded library molecules were mixed with the BI-occupied β2AR Nanodiscs immobilized on NeutrAvidin beads through biotin–avidin interaction of biotinylated membrane scaffolding protein ApoA1. Three rounds of iterative selection were performed with each library. (B) Structures of the Cmpd-6 and six other primary hits. These compounds have varied chemical scaffolds in a common region, designated as R1. The different chemical structures in the R1 region of each analog are illustrated. (C) Analysis of Cmpd-6 for its physical interaction with the agonist-bound, active β2AR by ITC. The thermogram (insert) and binding isotherm with the best titration curve fit are shown. One site model was used to fit the data. Data are representative of three independent experiments. The values summarizing binding affinity (KD), stoichiometry (N), enthalpy (ΔH), and entropy (ΔS) are shown in box below the graph.