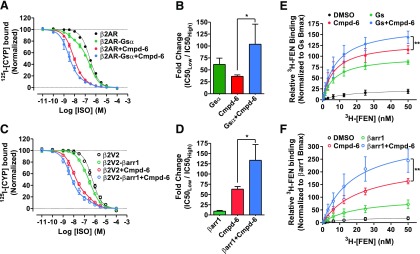
Fig. 5.
Positive allosteric cooperativity of Cmpd-6 for transducer-induced activities at the β2AR. (A–D) Data showing positive cooperativity of Cmpd-6 (20 µM) assessed by 125I-CYP versus (ISO) competition binding at membrane preparations from HEK cells overexpressing β2AR or the transducer fusions β2AR-Gsα (A) and β2V2R-β-arrestin1 (βarr1) (B). Points on the curves represent normalized cpm values from three independent experiments, expressed as two-site curve fit with shared IC50Low (GraphPad Prism). Associated bar graphs show Cmpd-6–mediated fold changes in ISO affinity at β2AR-Gsα (B), and β2V2R-βarr1 (D) fusions, respectively, expressed as a ratio of IC50Low/IC50High. (E and F) 3H-FEN saturation-binding curves showing Cmpd-6–mediated potentiation 3H-FEN binding at Sf9 cell membranes expressing the β2AR (E) and in cells expressing the phosphorylated β2V2R (F). Points on the curves represent cpm values normalized to the maximal level mediated by Gs (E) or βarr1 (F), respectively. DMSO (0.2%) was included as vehicle control in respective experiments for conditions without Cmpd-6. Values indicate mean ± S.D. from at least three independent experiments. Statistical analyses for the results depicted as the bar graphs (B and D) as well as Bmax changes in (E and F) were performed using one-way analysis of variance, repeated (related) measures with Tukey’s multiple comparison post-tests. Adjusted *P < 0.05; **P < 0.01.